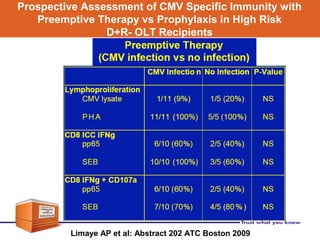
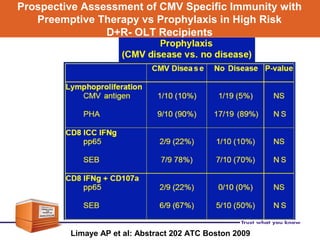
Valganciclovir is a prodrug of ganciclovir used for CMV prophylaxis and treatment. A key study (PV16000) found that valganciclovir prophylaxis for 100 days was as effective as oral ganciclovir for preventing CMV disease in high-risk transplant patients. Valganciclovir had a higher bioavailability than oral ganciclovir but was associated with a higher rate of neutropenia as an adverse effect. Long-term follow up of patients in the VICTOR study found rates of CMV disease recurrence after stopping prophylaxis of 15.1% at 1 year. Factors like viral load at baseline and