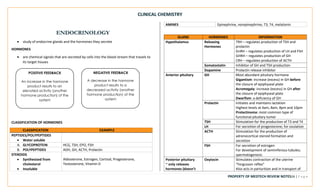
This document provides an overview of key terms and concepts related to clinical chemistry quality control. It defines common quality control terms like quality, control, standard, specificity, sensitivity, accuracy, and precision. It also describes different types of quality control, including intralaboratory quality control to detect random and systematic errors, and interlaboratory quality control to maintain long-term accuracy through comparison with other laboratories. Common quality control charts and rules for identifying errors are discussed. Key instrumentation and analytical techniques used in clinical chemistry are briefly outlined, including spectrophotometry, atomic absorption spectrophotometry, flame emission photometry, fluorometry, turbidimetry, nephelometry, potentiometry, electrophoresis, and chromatography.




















![CLINICAL CHEMISTRY
PROPERTY OF MEDTECH REVIEW NOTES22 | P a g e
BLOOD GAS
DEFINITION OF TERMS:
• Acid: a compound that could donate a H+ ion
• Base: a compound that could accept a H+ ion
• Acid-Base Balance: a mechanism by which the pH of blood is maintained at 7.35-
7.45 for homeostasis
• Buffer: a weak acid/base with its conjugate salt that resists changes in Ph
ACID BASE BALANCE
HENDERSON-HASSELBACH EQUATION
• Implicates the relationship between pH, and the two involved organs - lungs and
kidneys
𝑝𝐻 = 6.1 + 𝑙𝑜𝑔
𝐻𝐶𝑂3
𝑃𝐶𝑂2 𝑥 0.0307
EXPANDED FORM: 𝑝𝐻 = 6.1 + log
[𝑇𝐶𝑂2−(𝑃𝐶𝑂2 𝑥 0.03)]
𝑃𝐶𝑂2 𝑥 0.03
FOUR BASIC ABNORMAL STATES
Imbalance pH pCO2 H2CO3 HCO3 Primary
compensation
Seen in:
Respiratory
Acidosis
↓ ↑ ↑ N Kidneys retain
bicarbonate & excrete
hydrogen
Pneumonia,
emphysema
Respiratory ↑ ↓ ↓ N Reverse of respiratory Hyperventilation,
Alkalosis acidosis early salicylate
poisoning
Metabolic
Acidosis
↓ N N ↓ Hyperventilate (CO2
blew off)
Diabetic
ketoacidosis,
renal disease
and prolonged
diarrhea
Metabolic
Alkalosis
↑ N N ↑ Hypoventilation (CO2
retention)
Vomiting,
antacids,
NaHCO3 infusion
EVALUATING ACID-BASE DISORDERS
1. Determine if the pH is high (alkalosis) or low (acidosis)
2. Compare pCO2 and HCO3 to normal values
✓ If pCO2 is opposite to pH = respiratory
✓ If HCO3 is in the same direction with pH = metabolic
3. If pH is within normal range, full compensation has occurred
4. if main compensatory mechanism has already occurred yet the pH is still out of
range, partial compensation happened.
NORMAL VALUES
• pH: 7.35 – 7.45
• pCO2: 35-45 mmHg
• pO2: 81-100 mmHg
• HCO3: 21-28 mEq/L
• TCO2: arterial (19-24 mmol/L); venous (22-26 mmol/L)
• H2CO3: 1.05-1.035 mmol/L
• O2 saturation: 94-100%](https://image.slidesharecdn.com/clinical-chemistry-v1-240127005142-f57c3350/85/Clinical-Chemistry-Must-Know-Notes-For-Medical-Technologist-pdf-22-320.jpg)