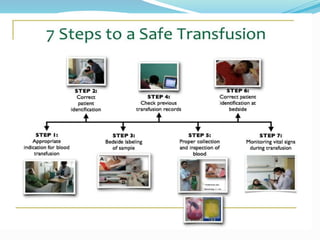
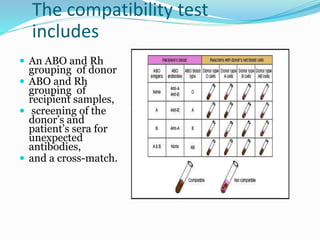
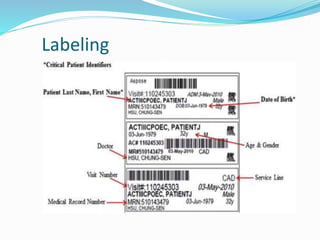
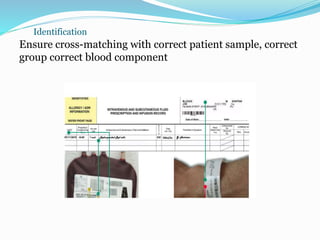
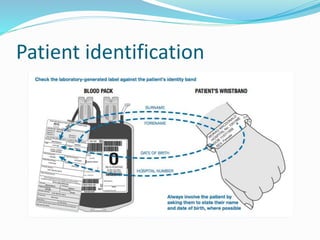
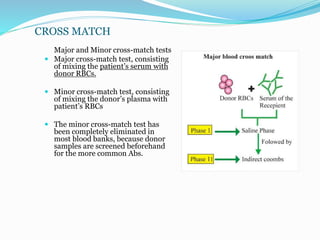
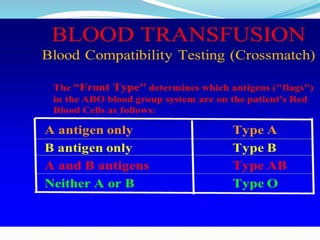
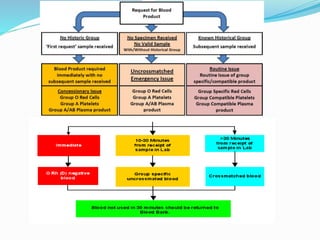
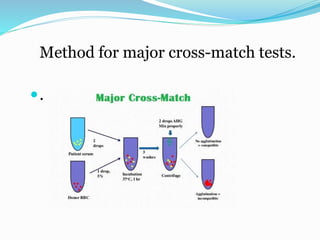
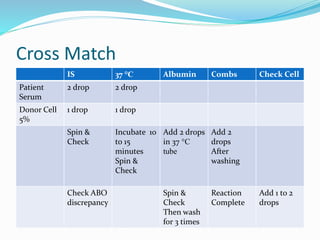
The document discusses the steps for compatibility testing prior to blood transfusion, which includes correctly identifying the donor and recipient, testing their blood samples, and performing a cross-match. It aims to detect any errors in grouping, labeling, or identification and to find any antibodies in the recipient's serum that could react with donor antigens to prevent adverse reactions.