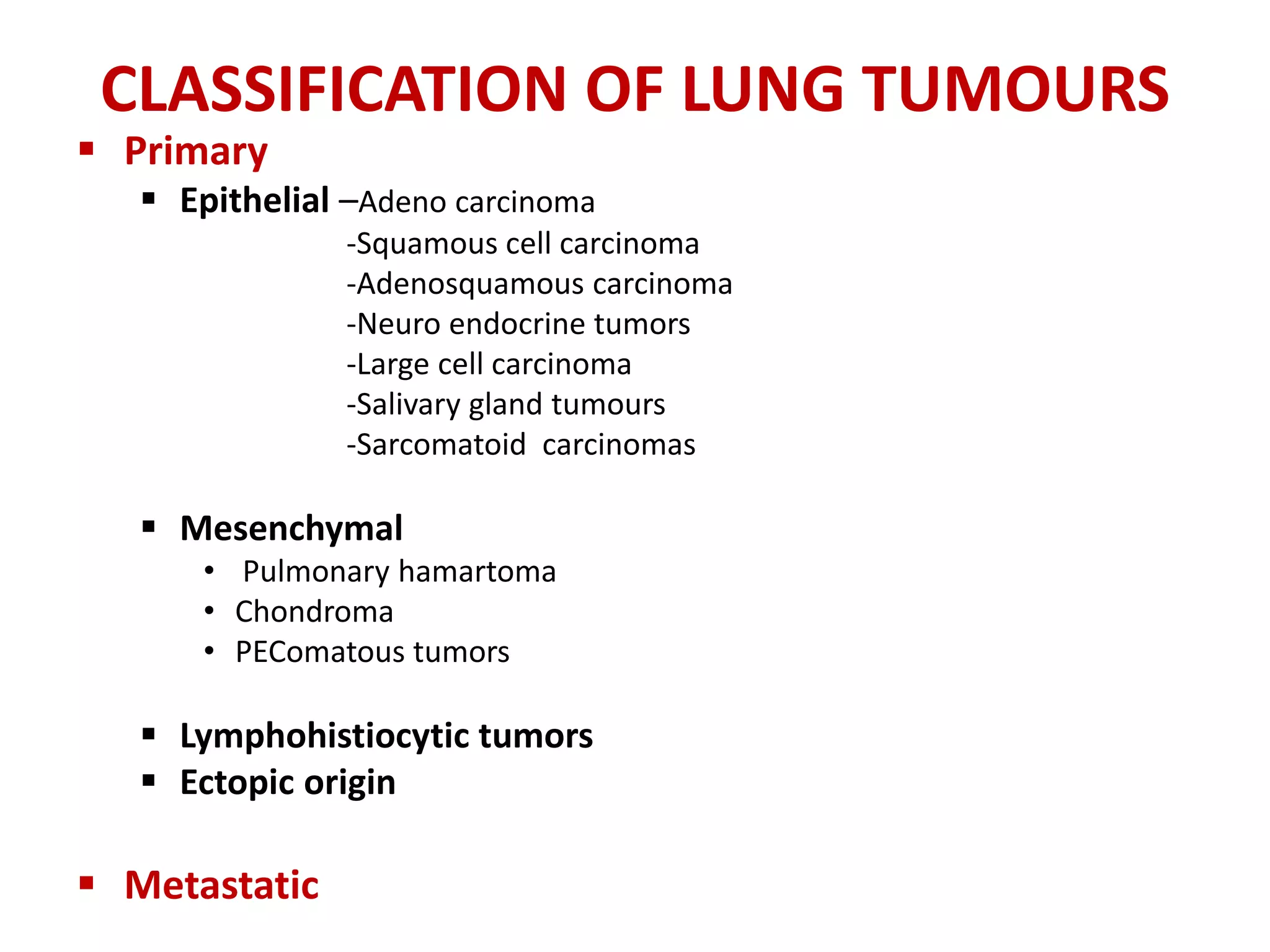
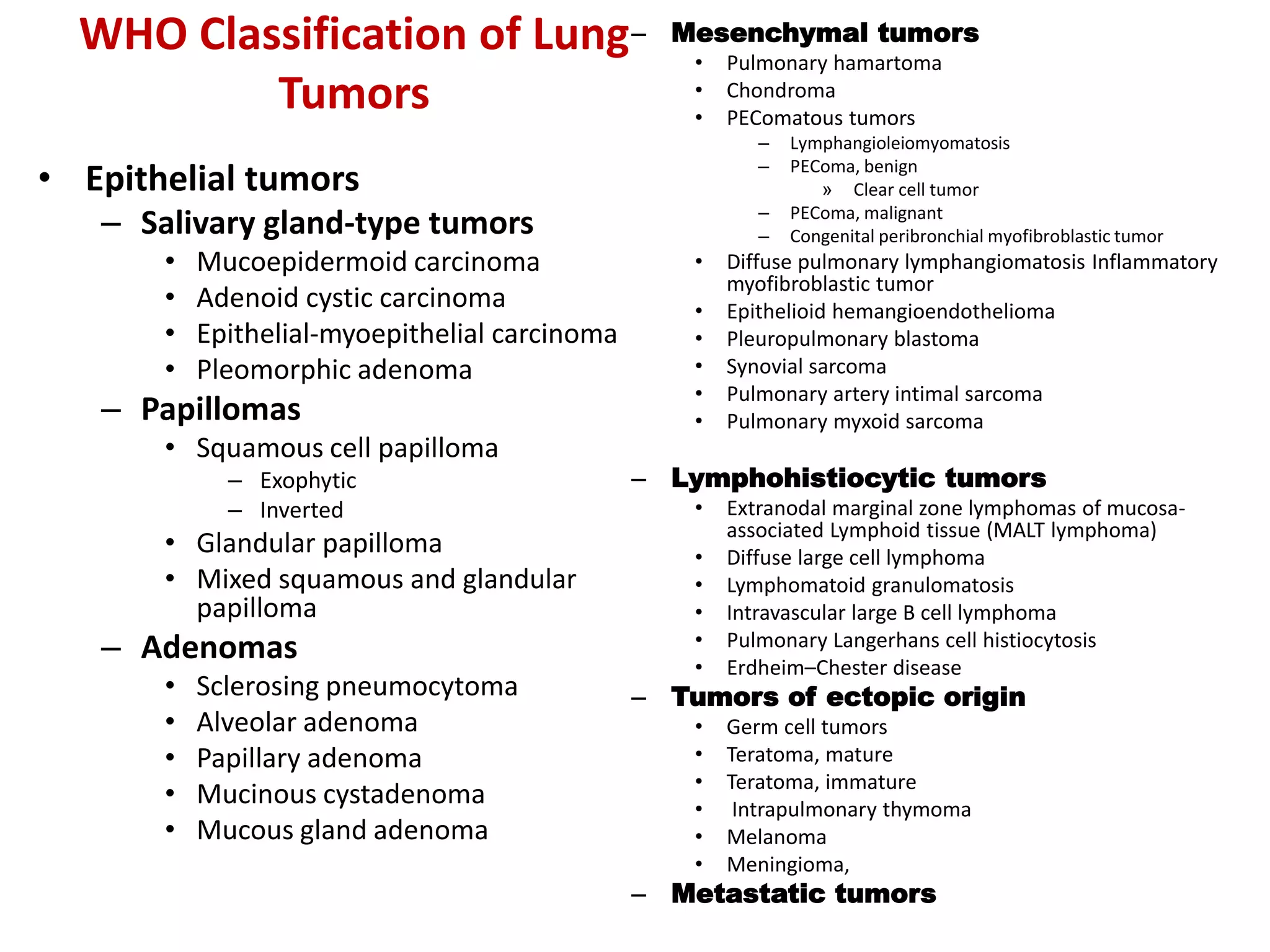
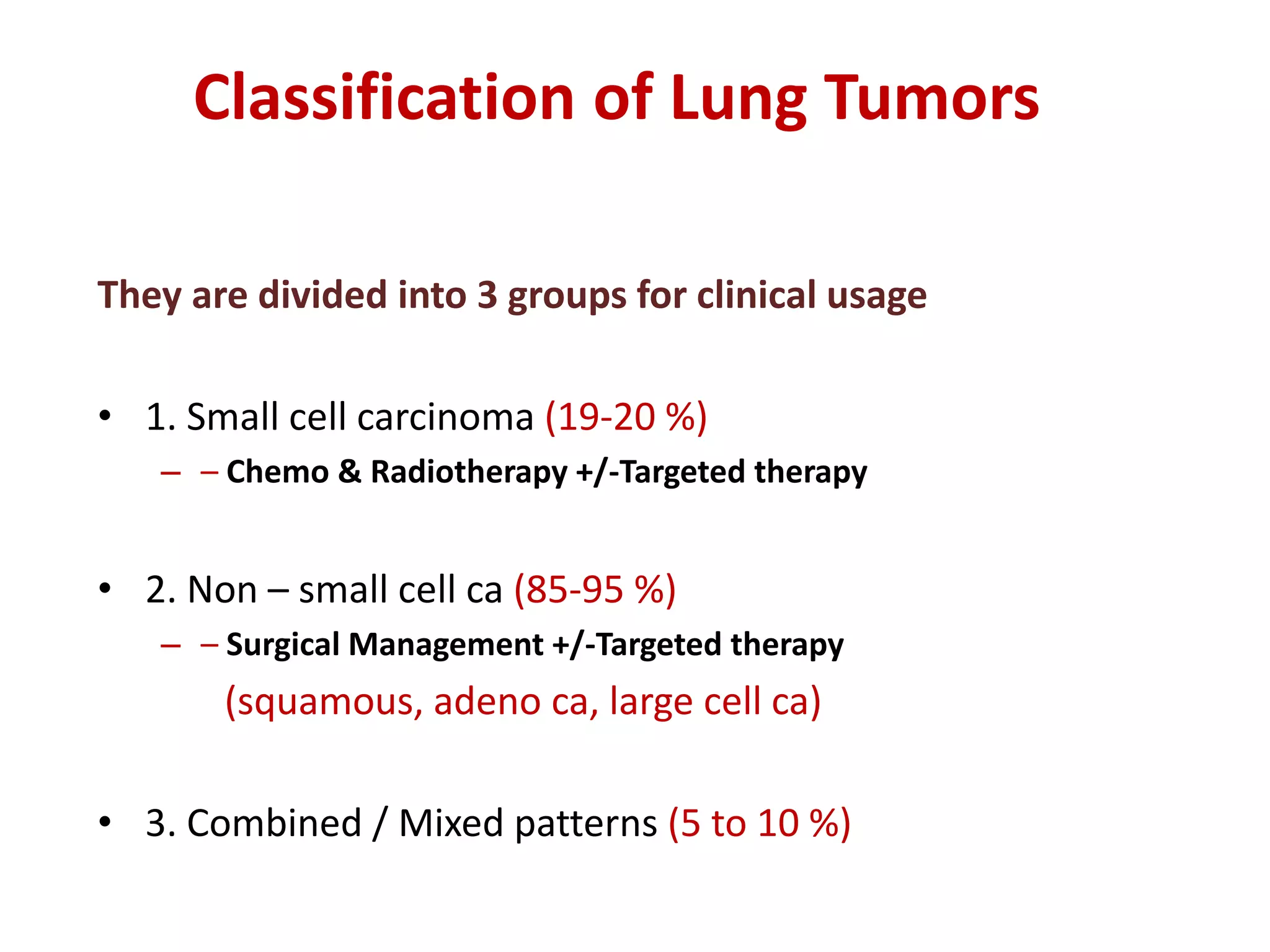
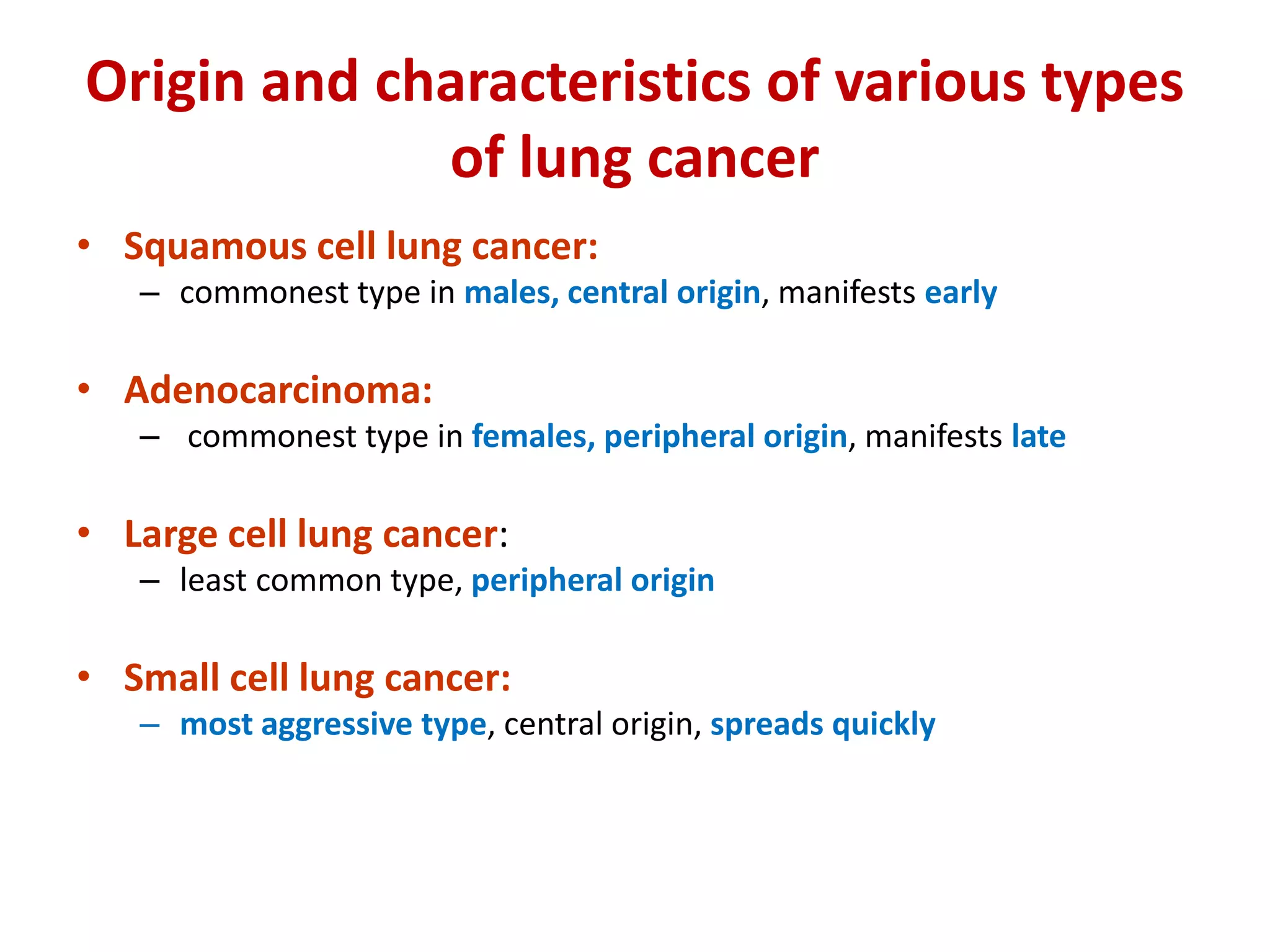
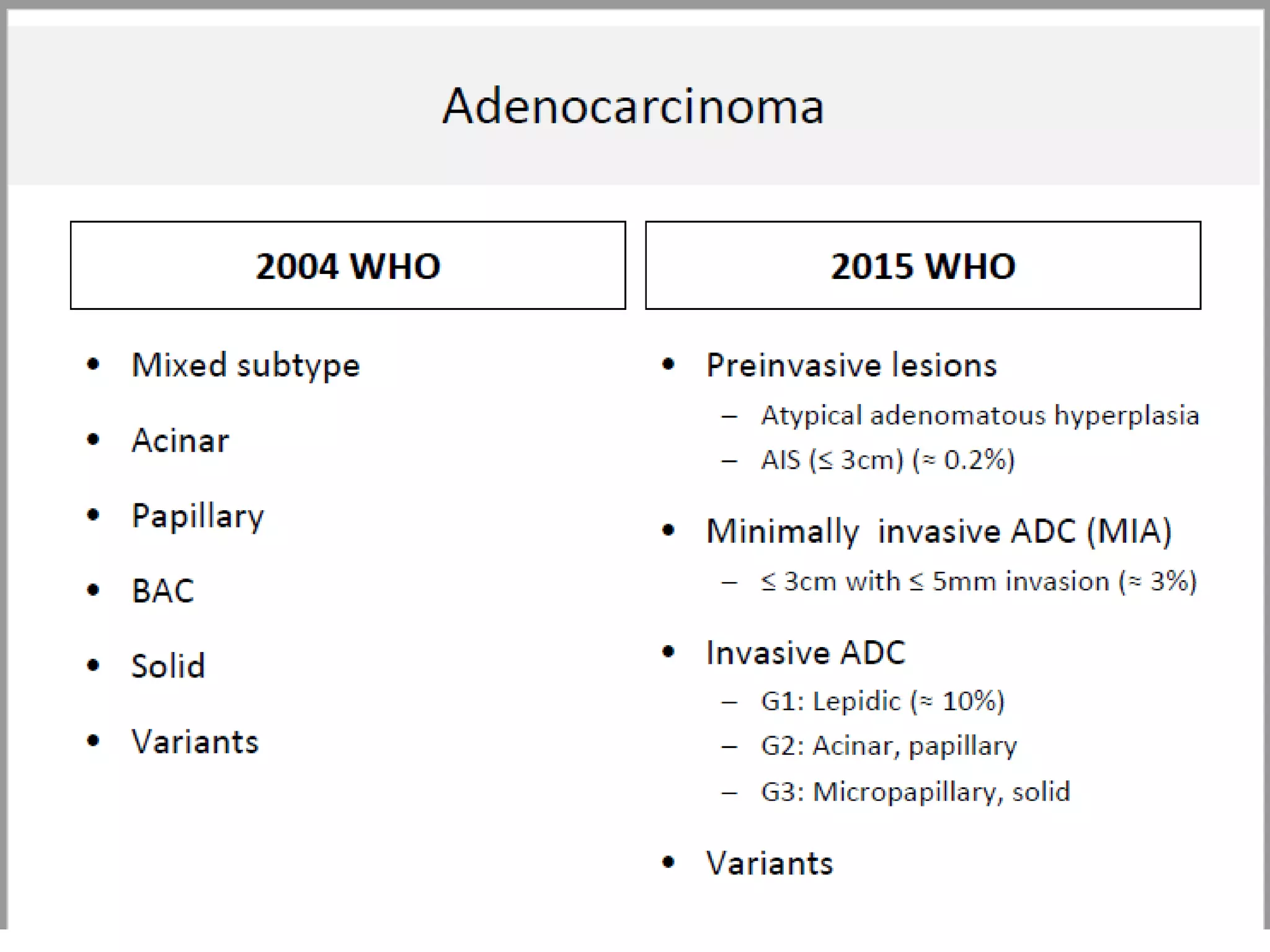
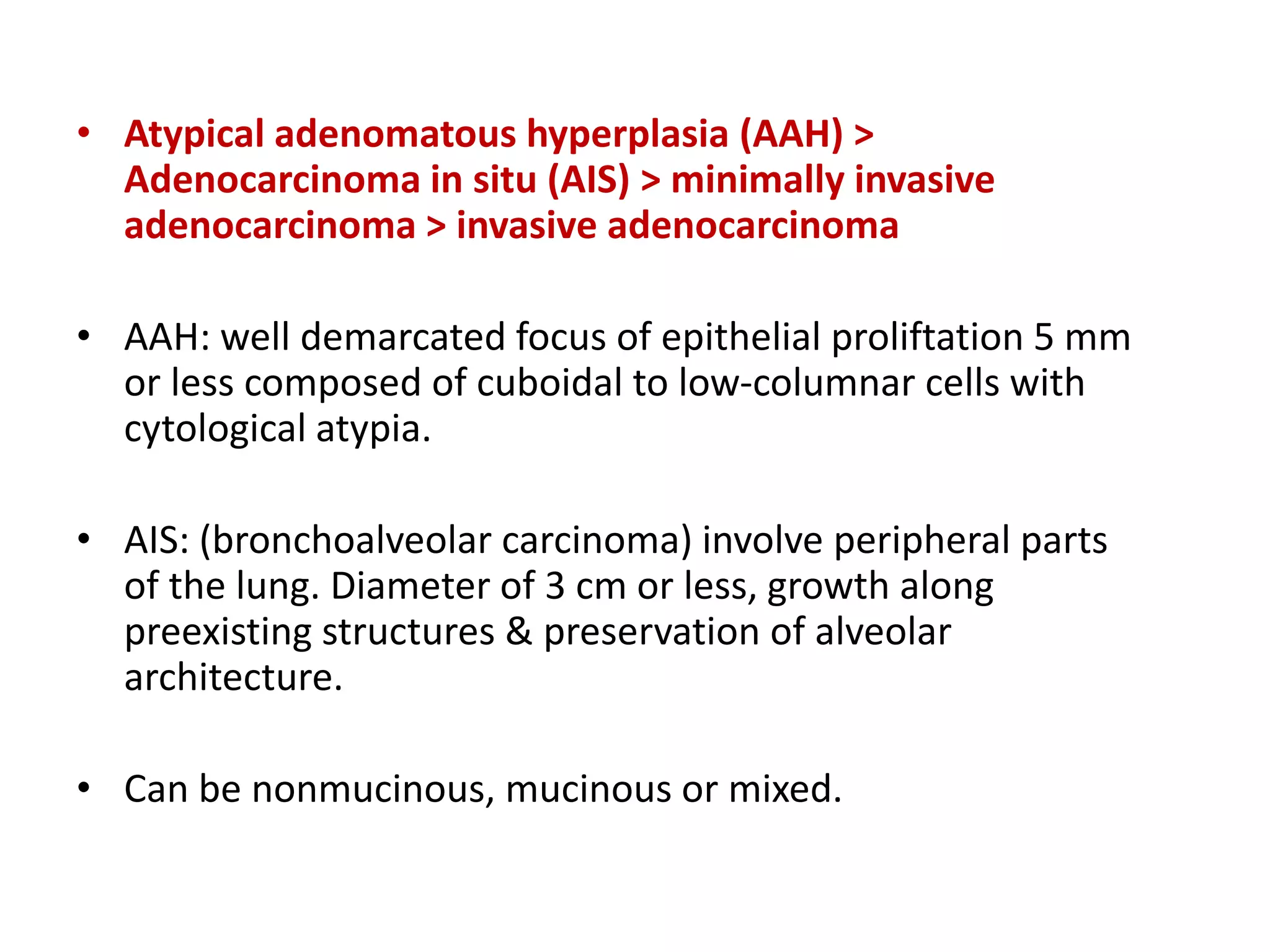
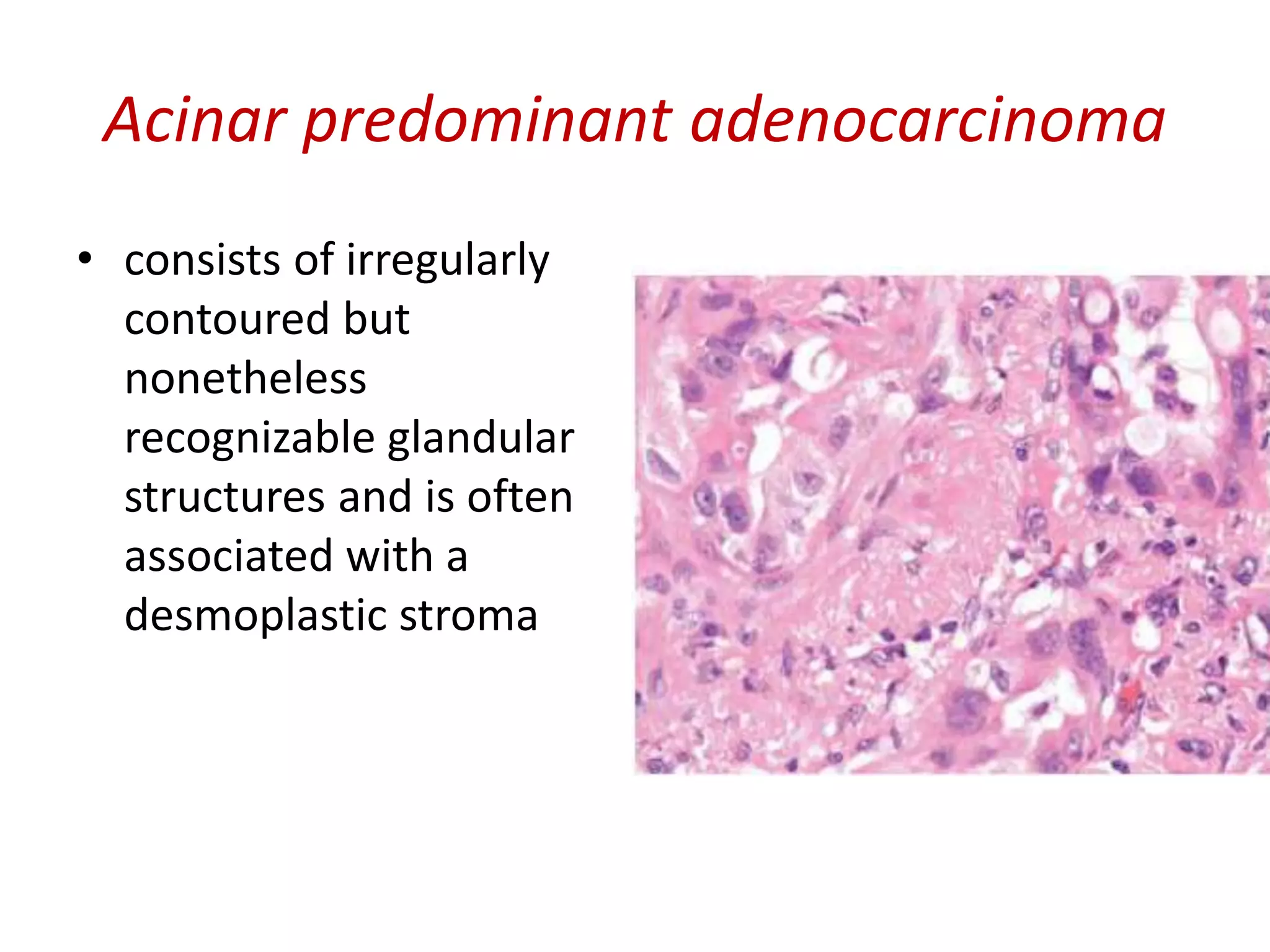
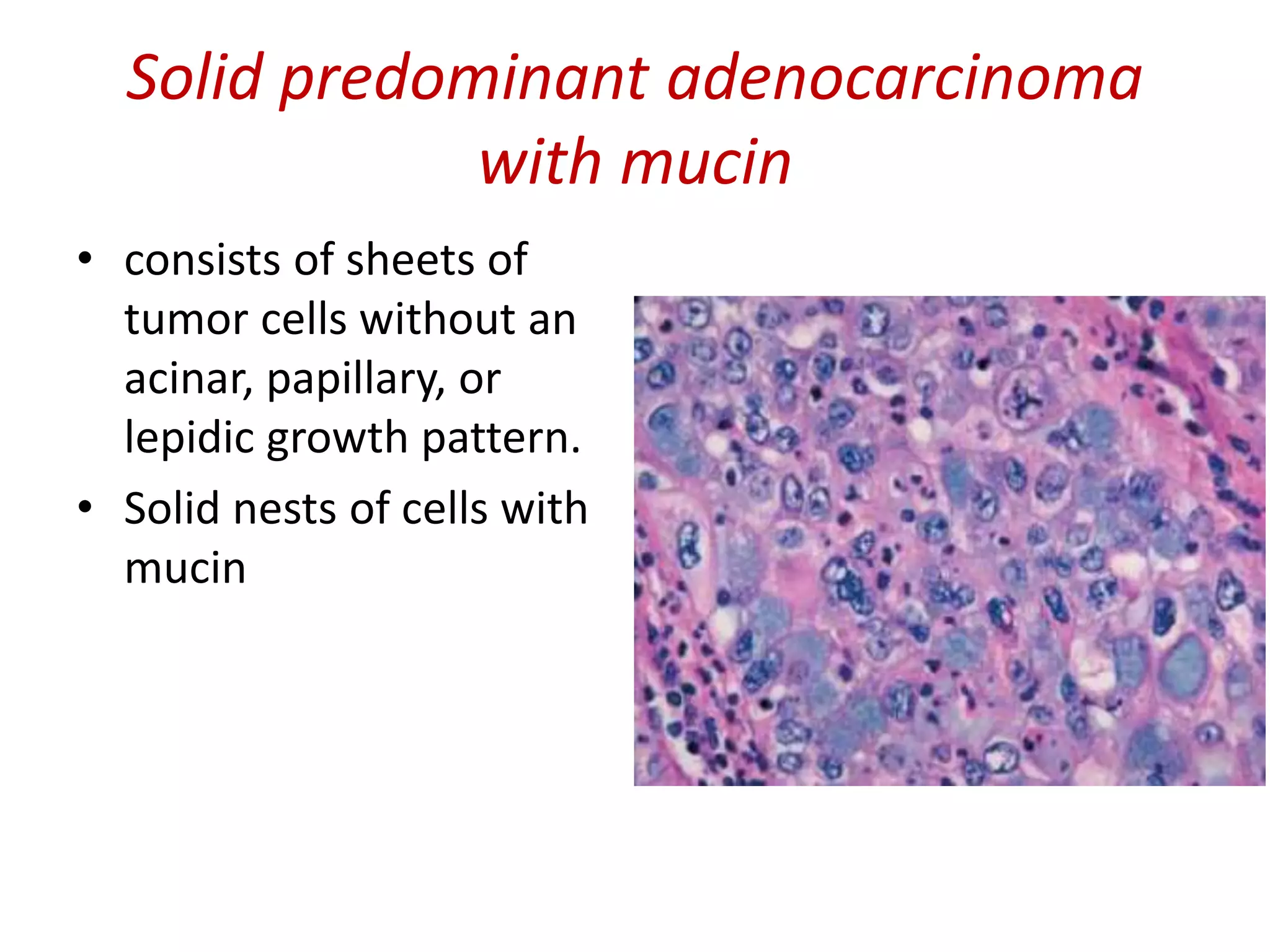
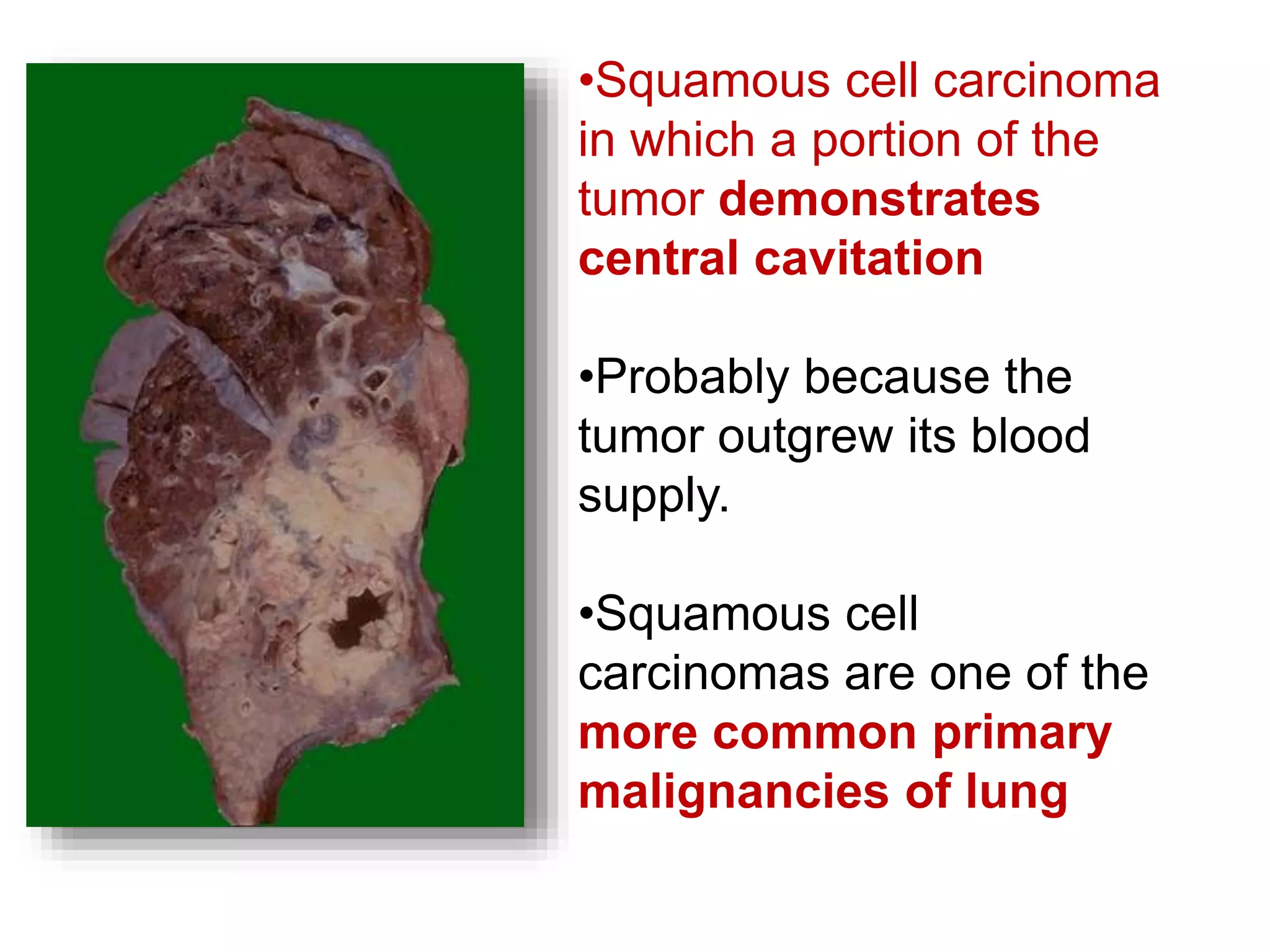
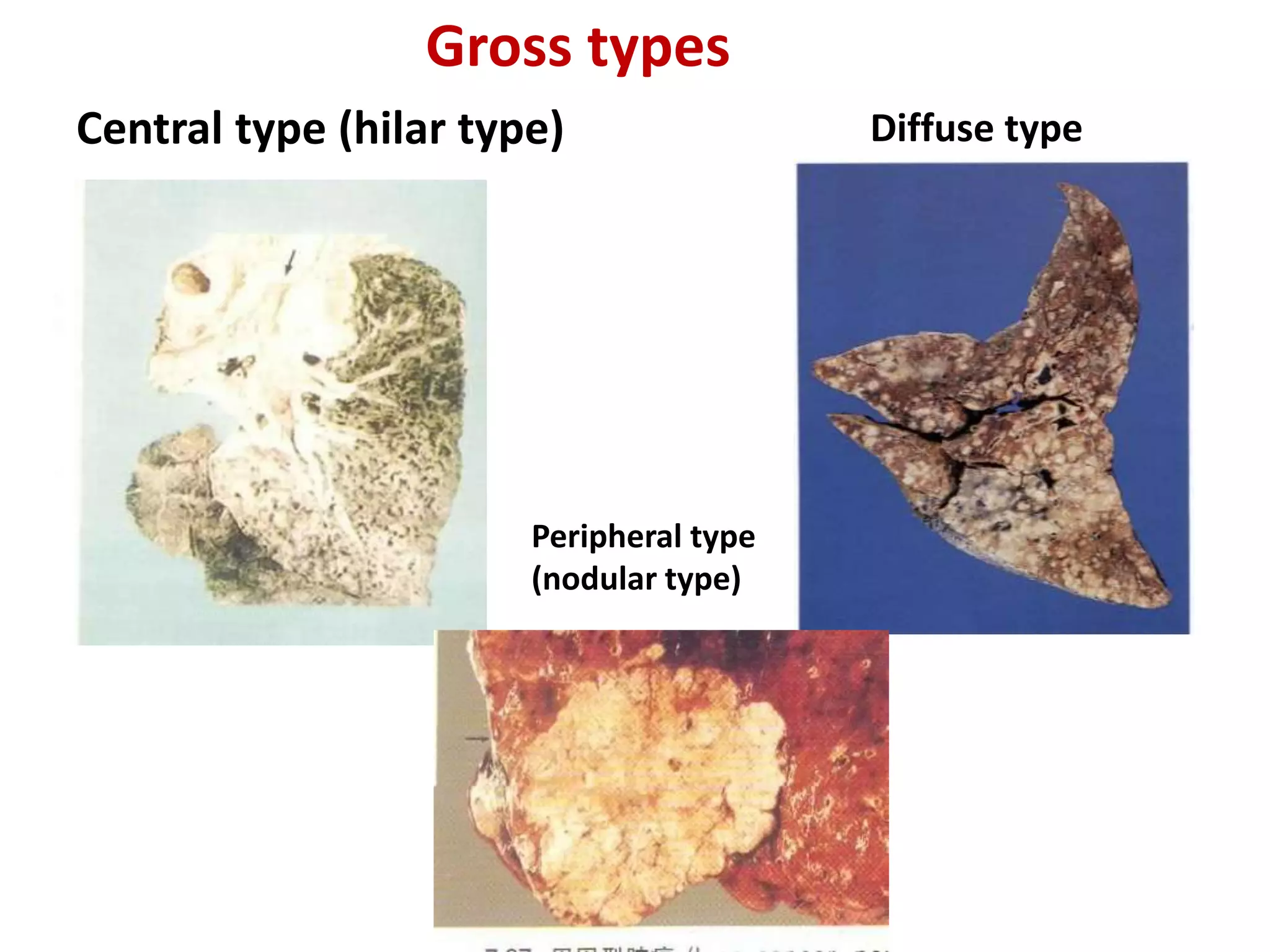
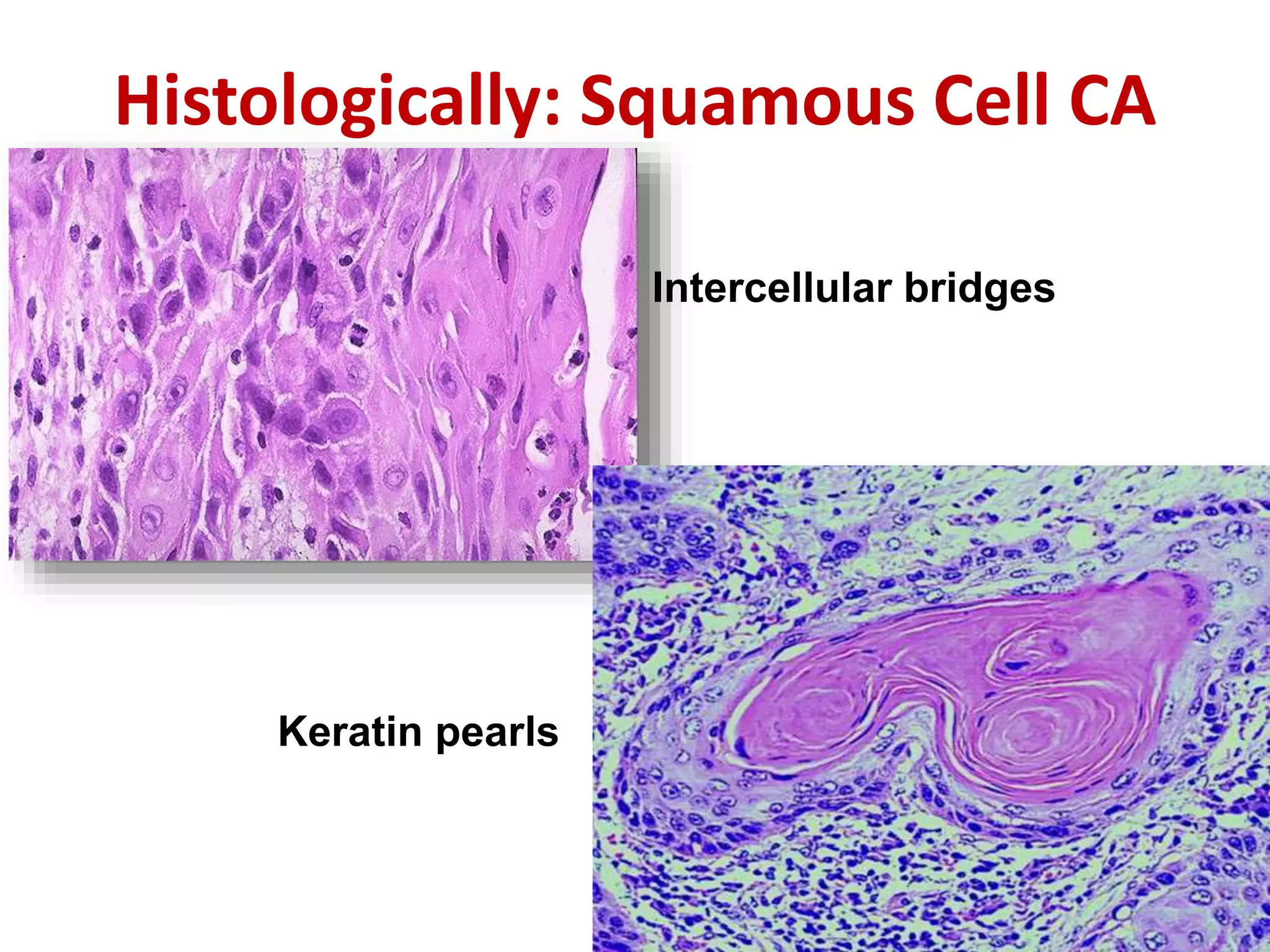
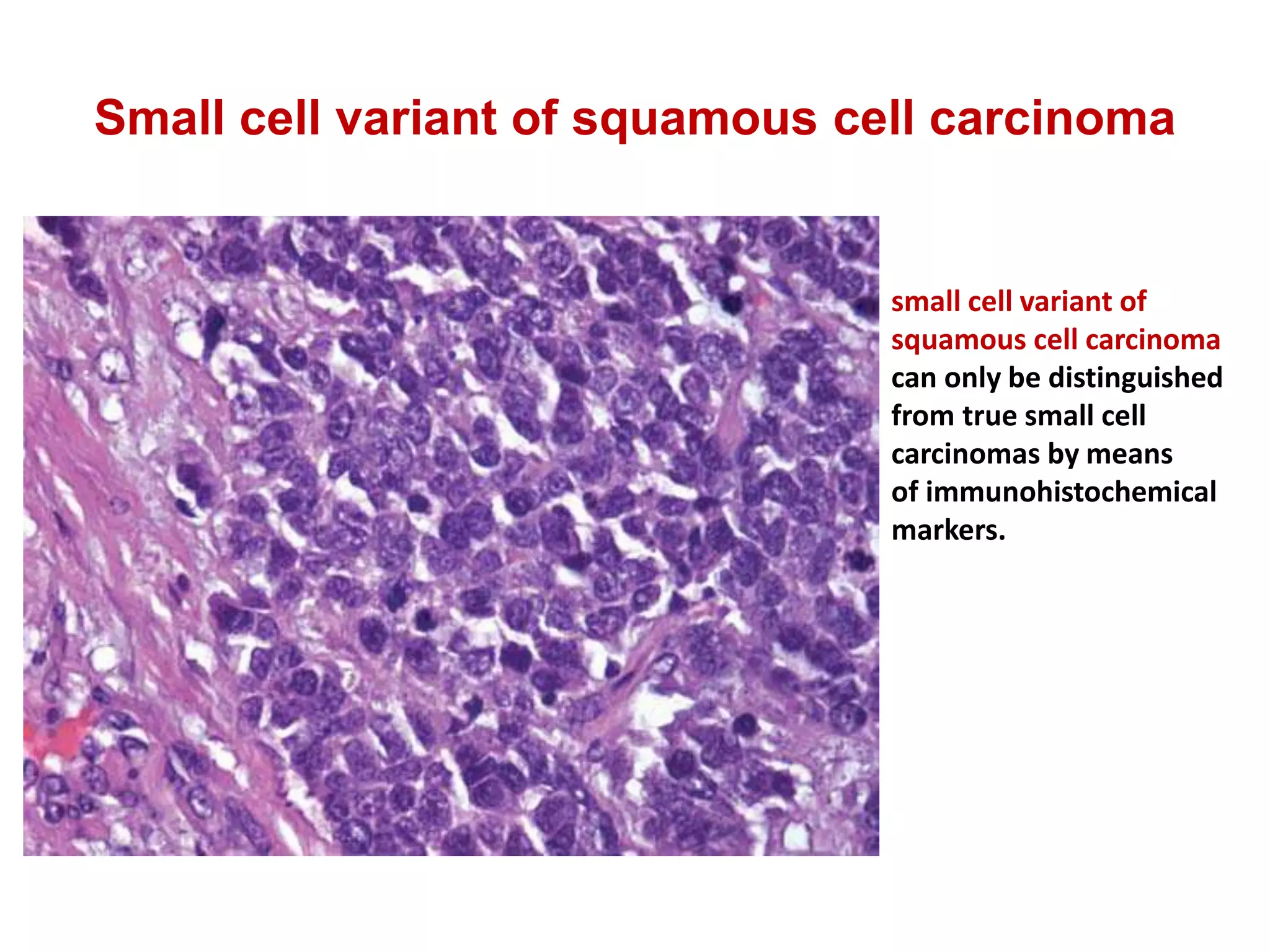
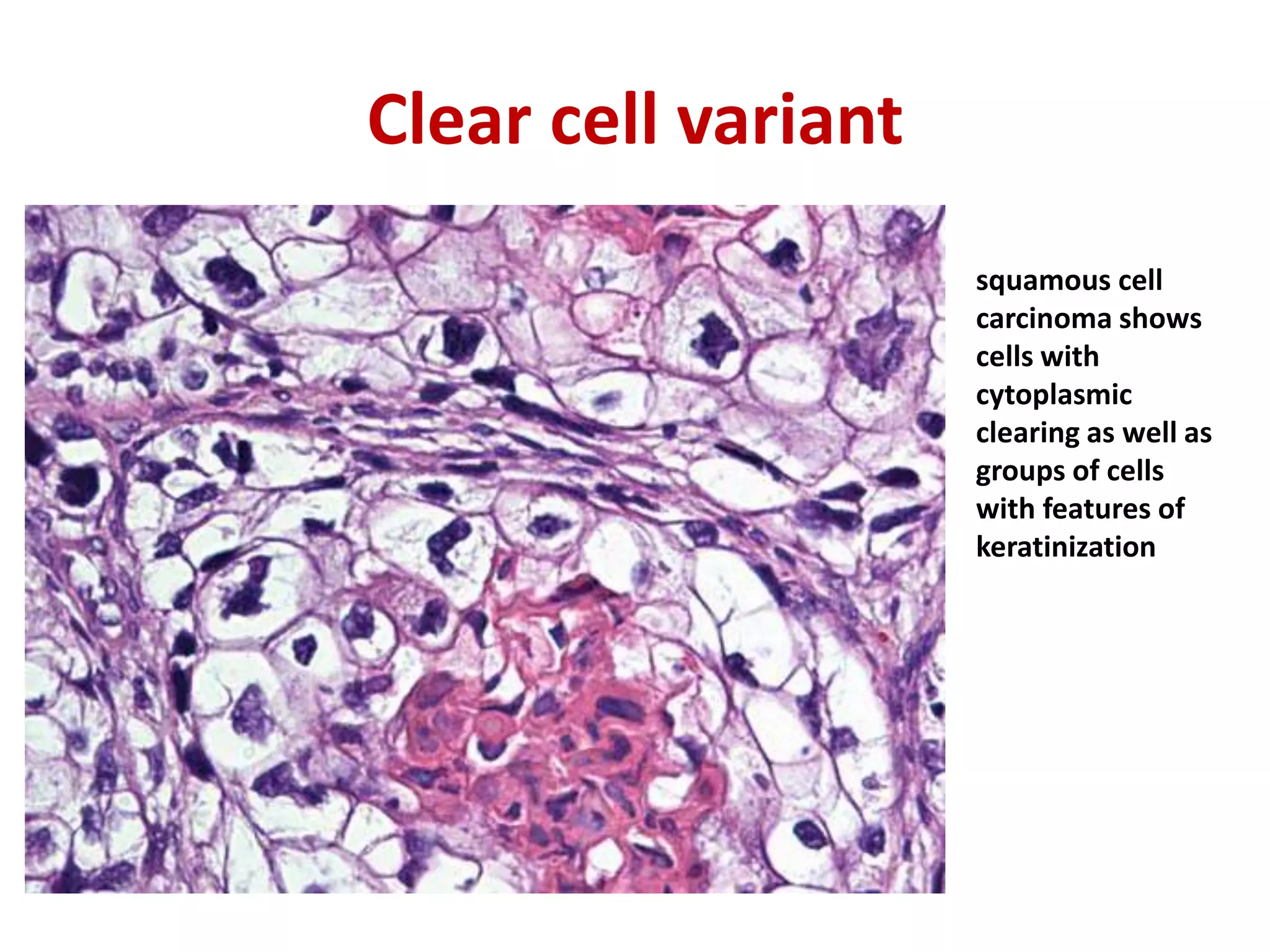
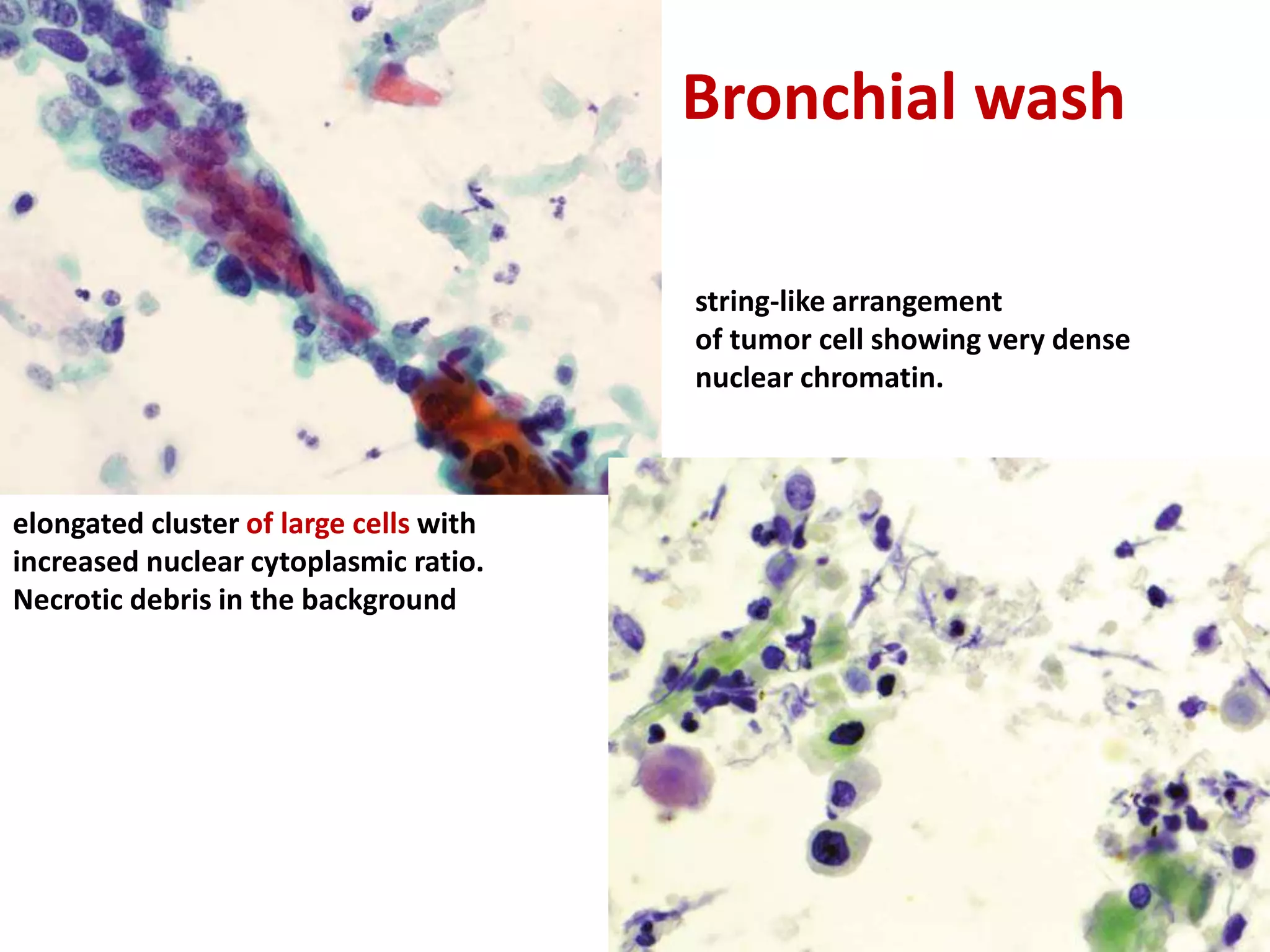
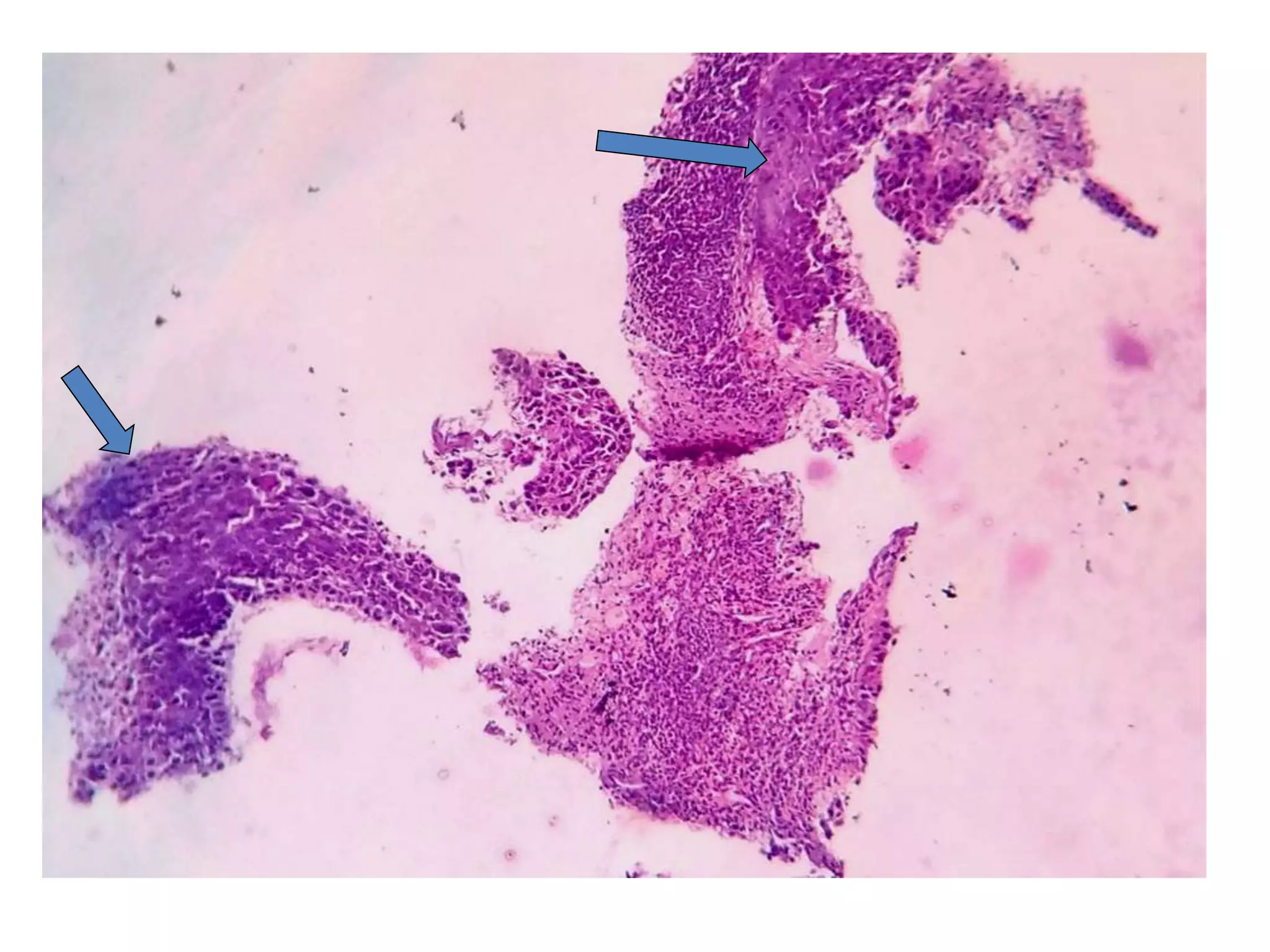
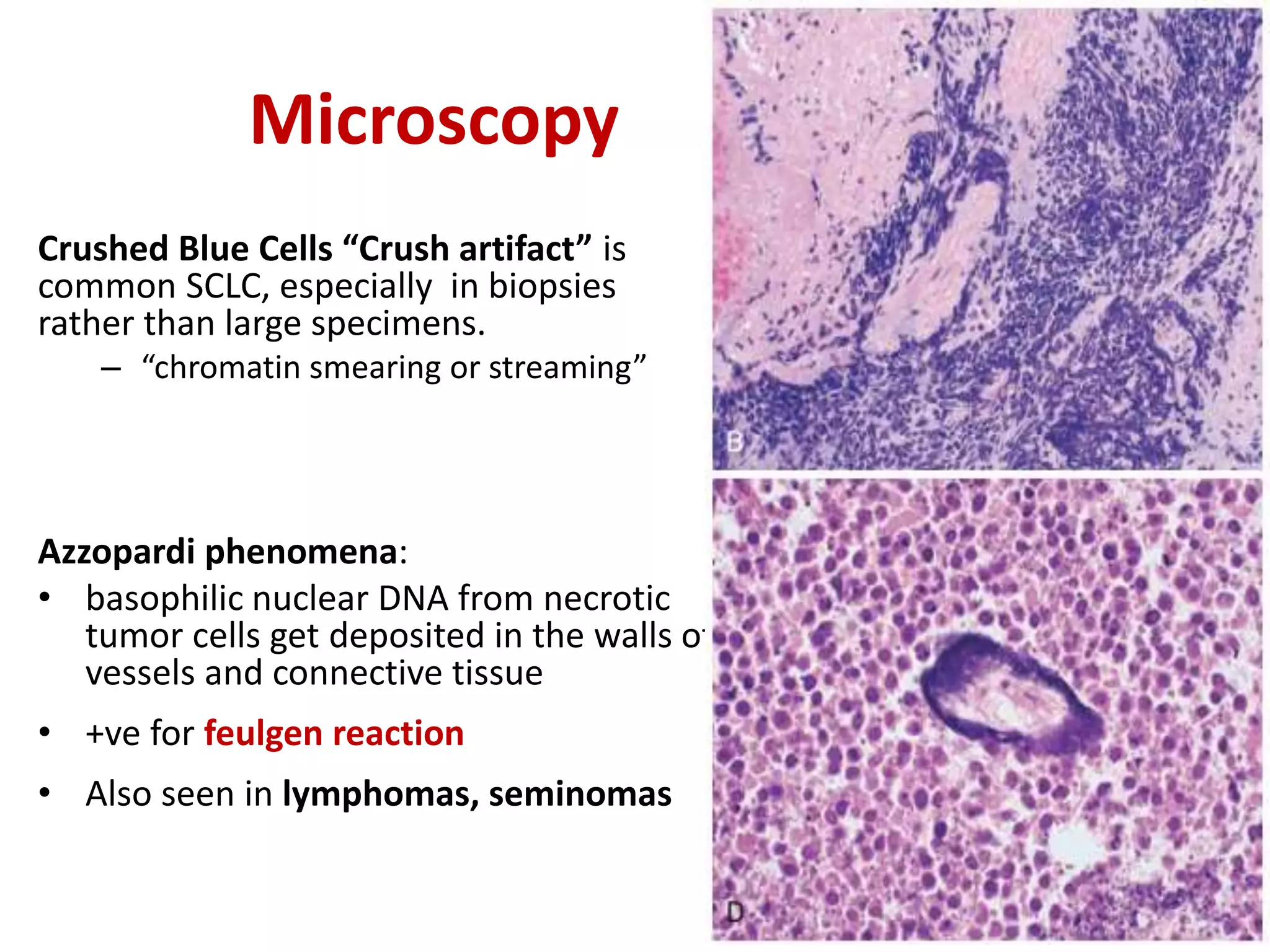
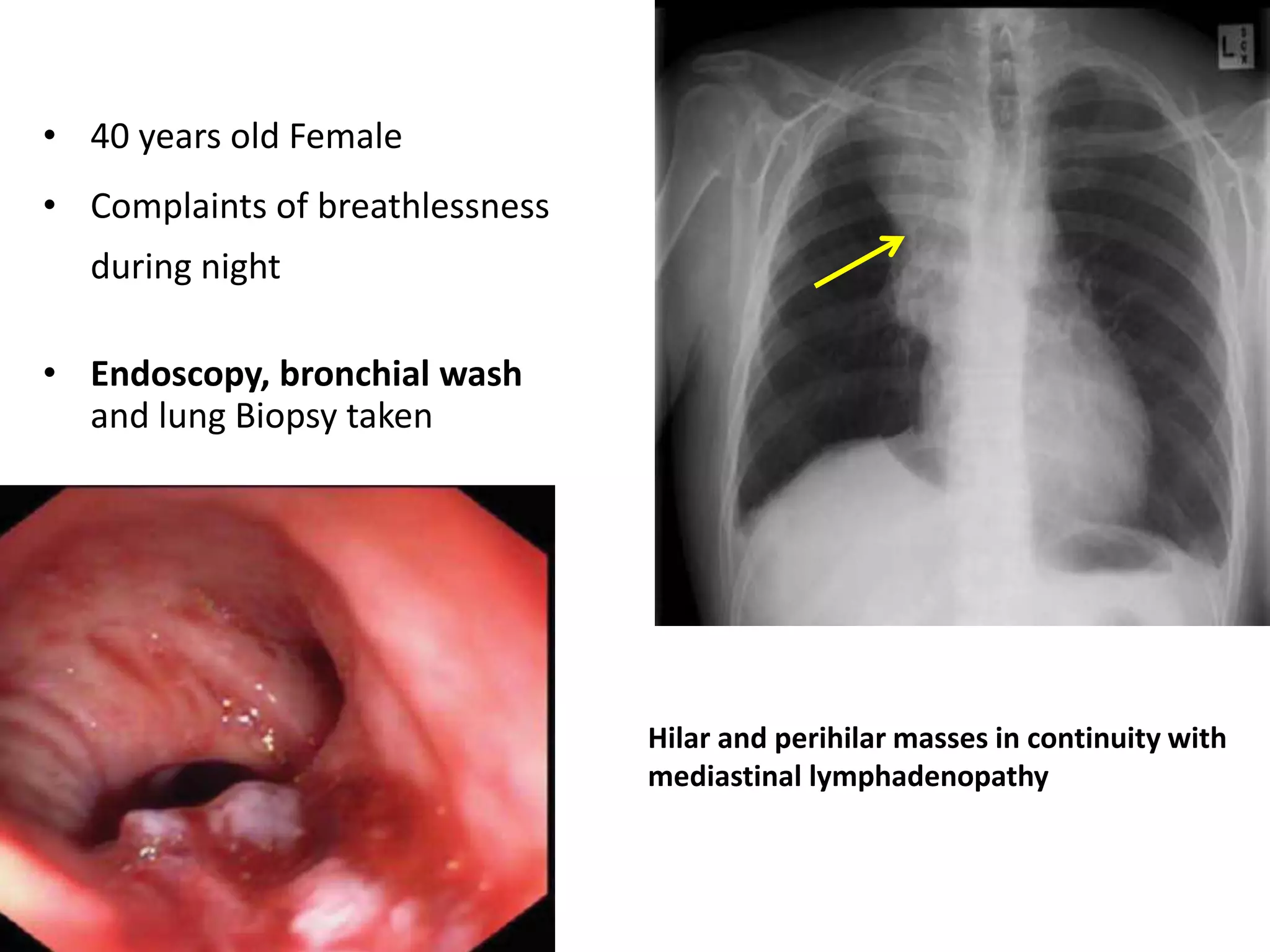
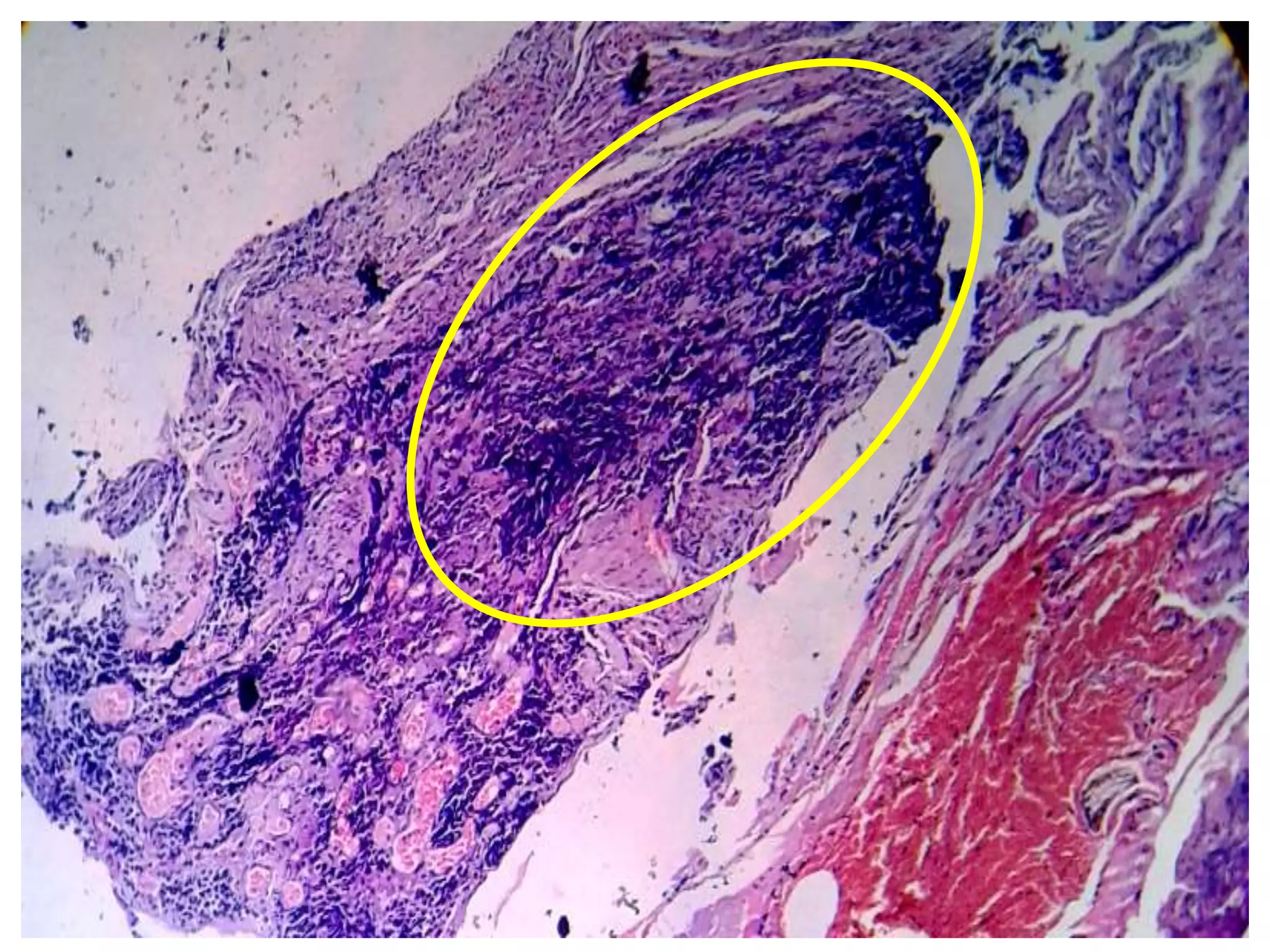
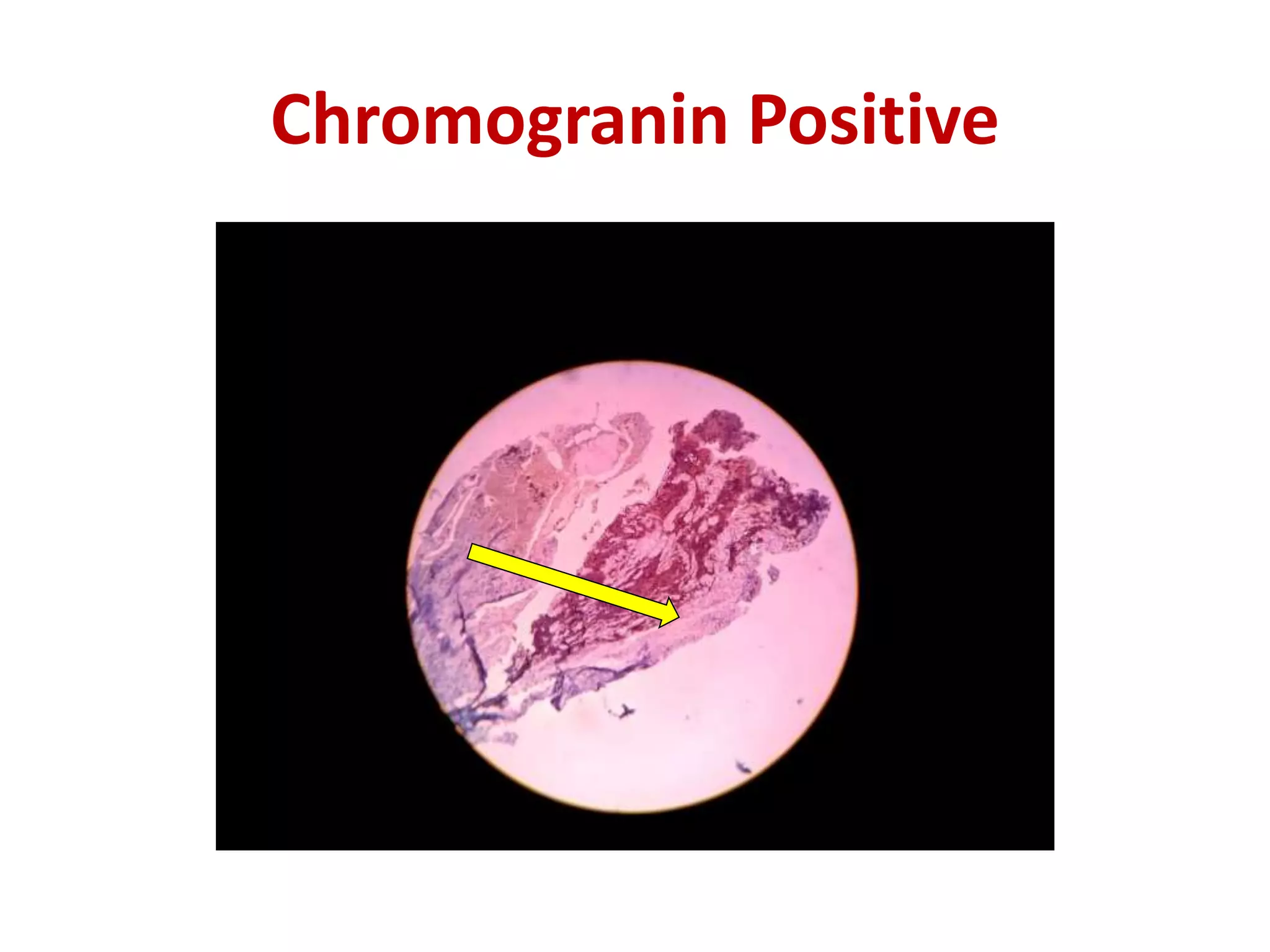
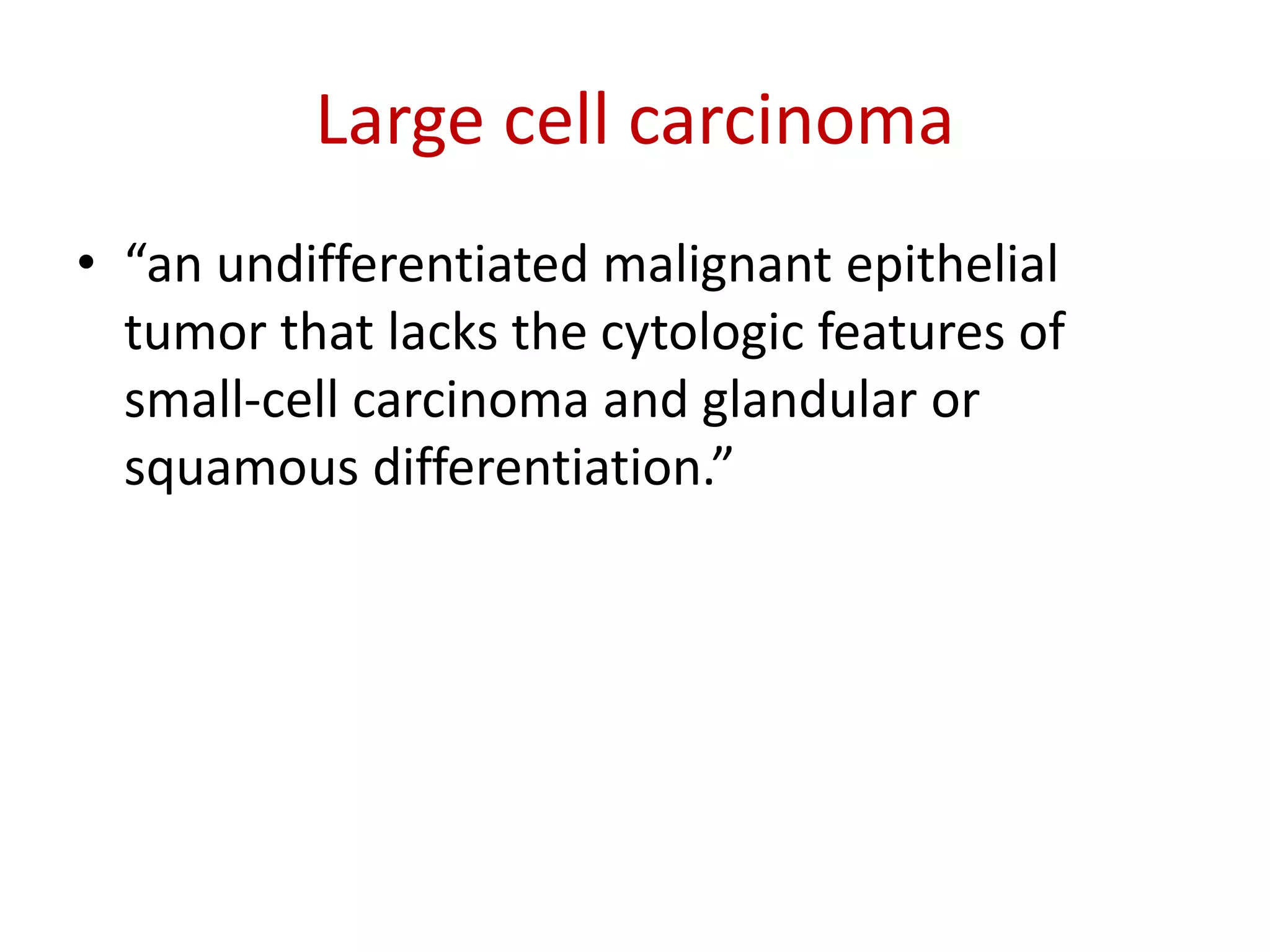
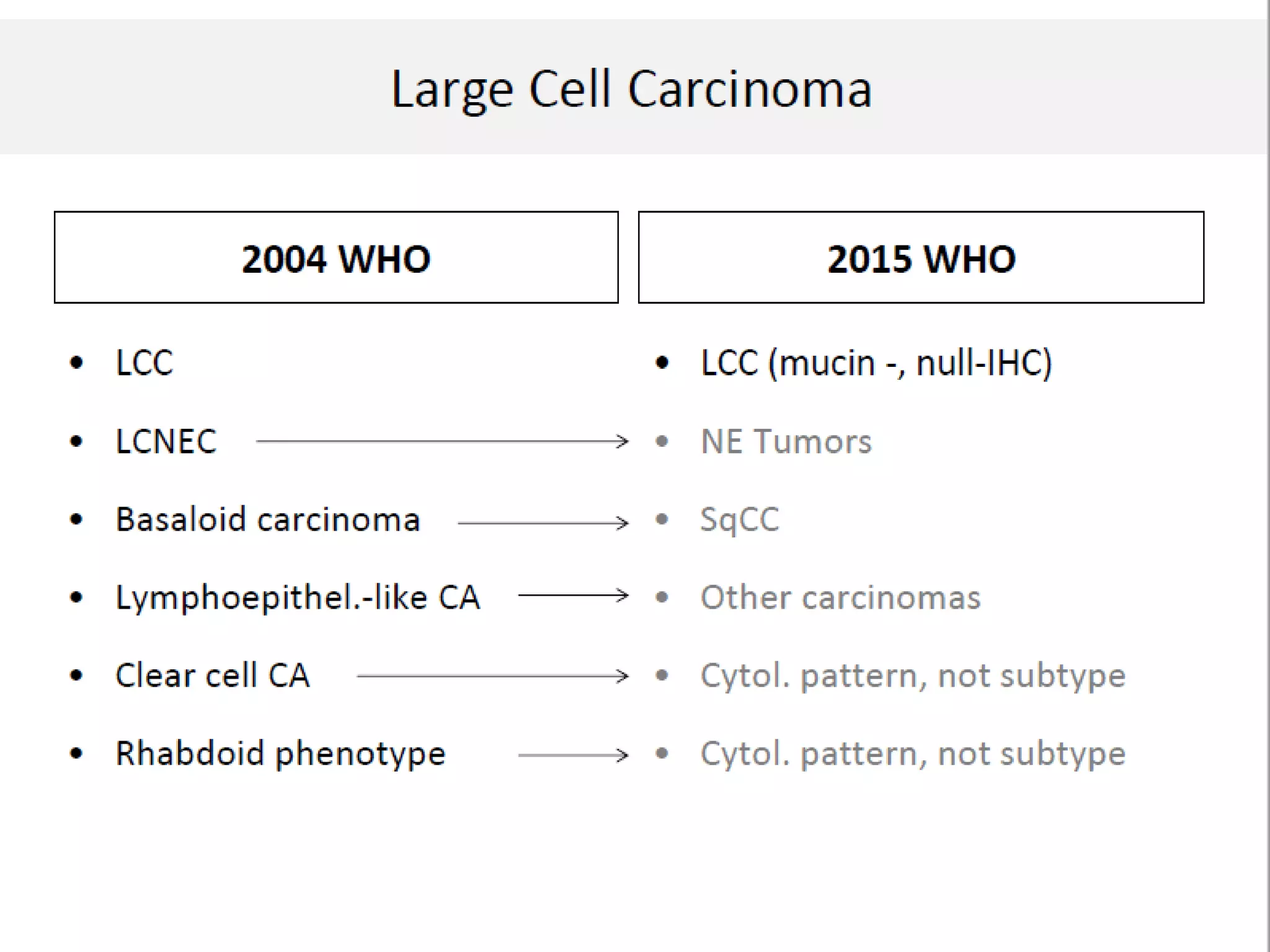
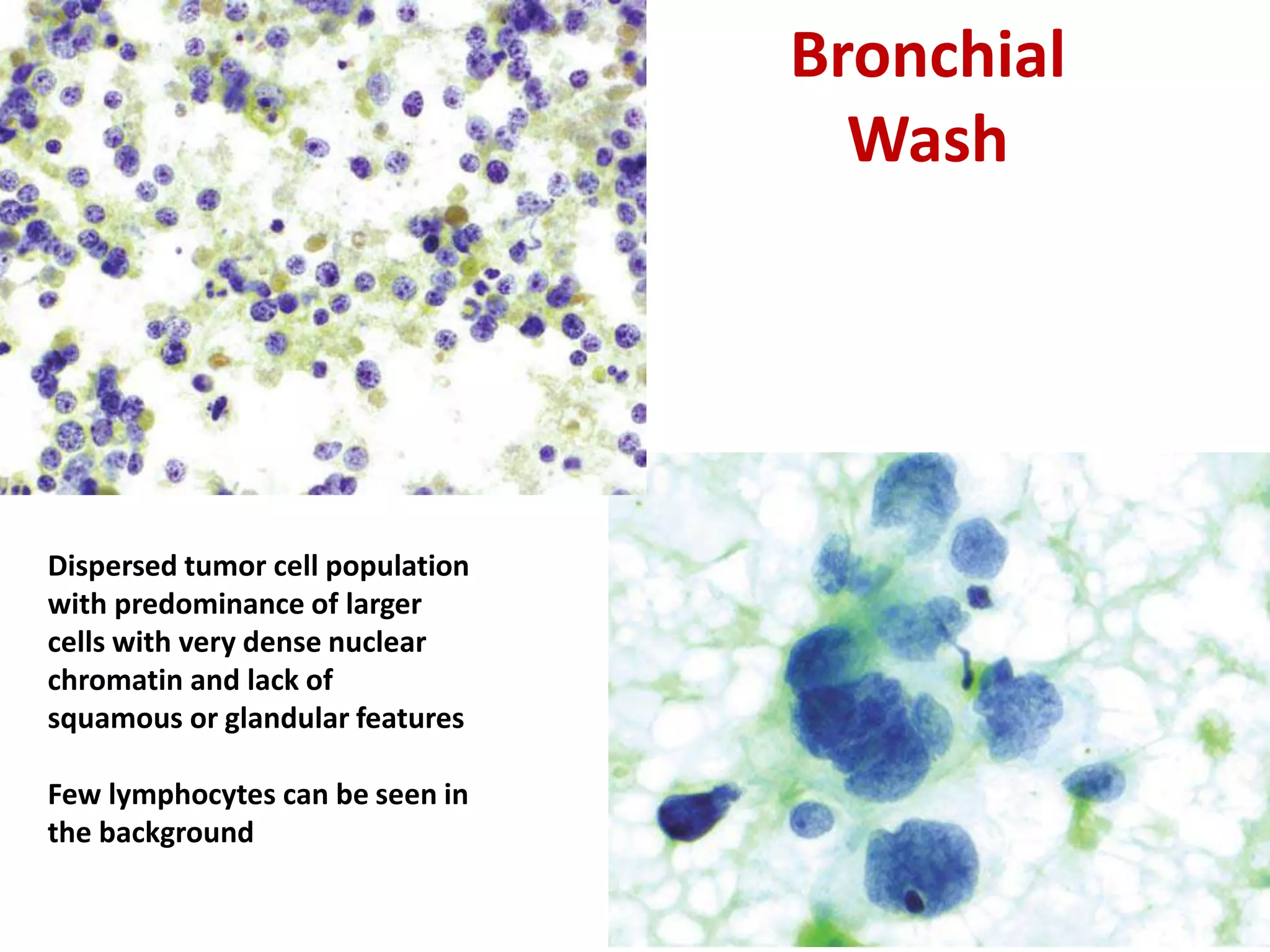
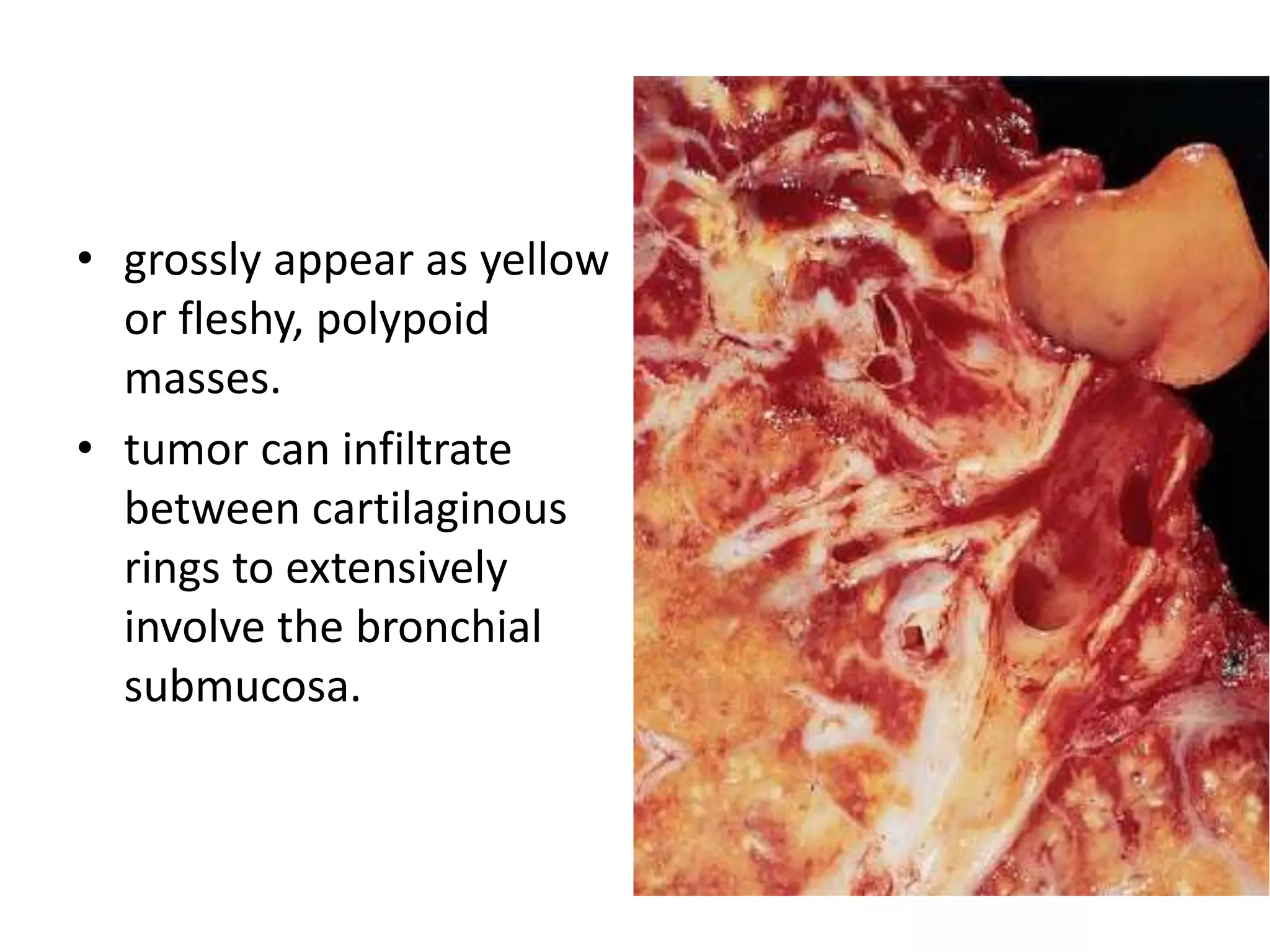
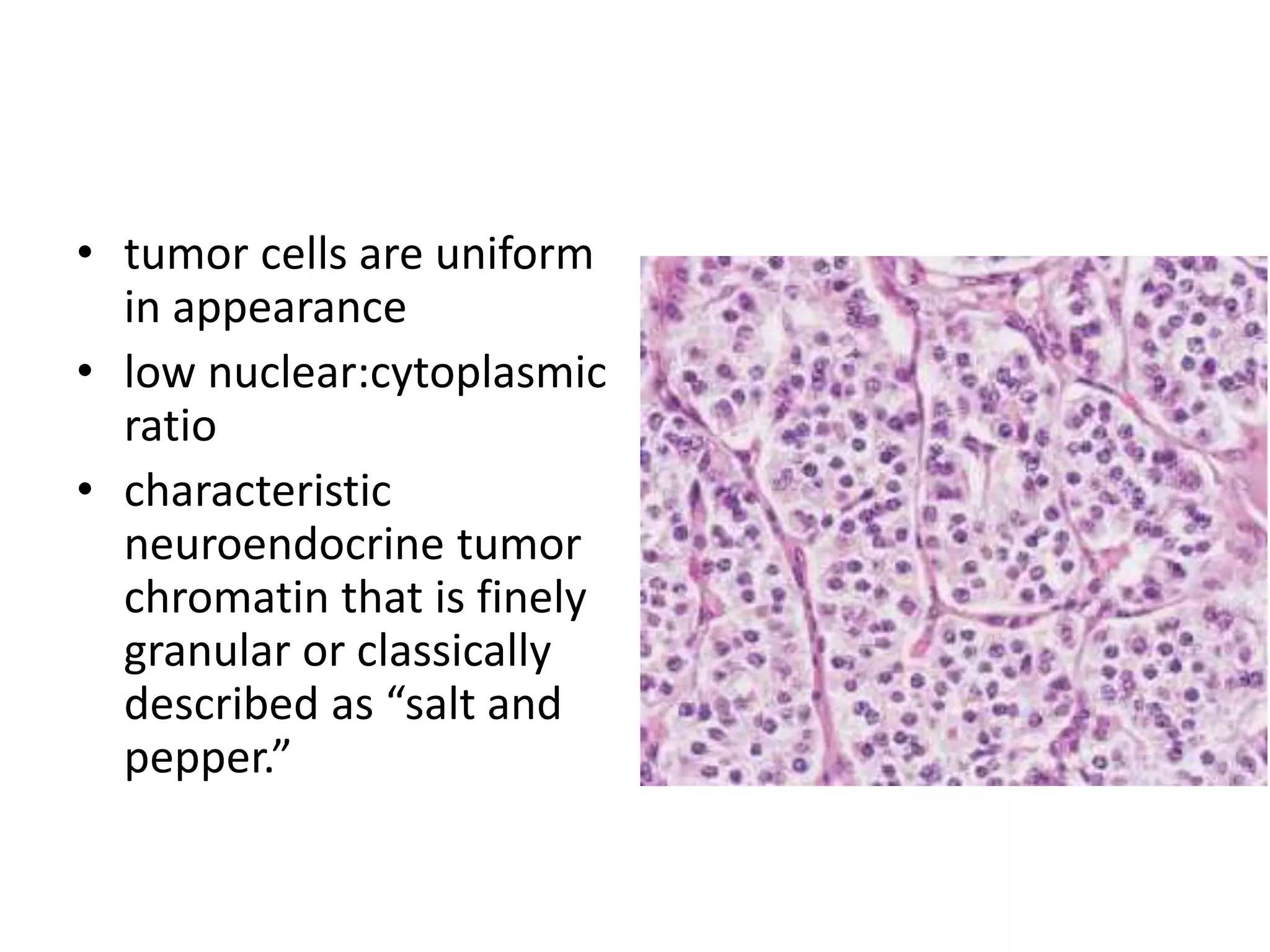
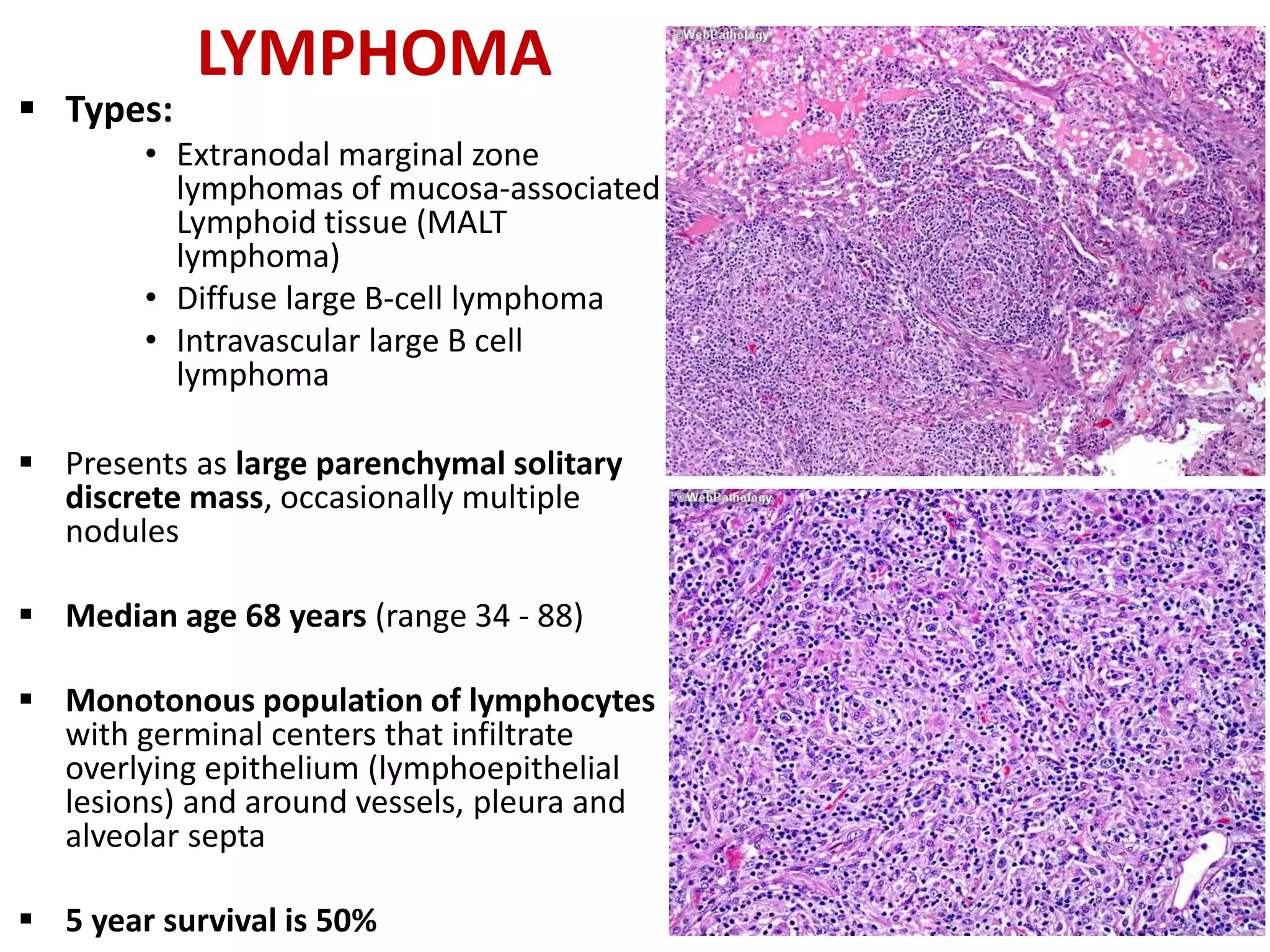
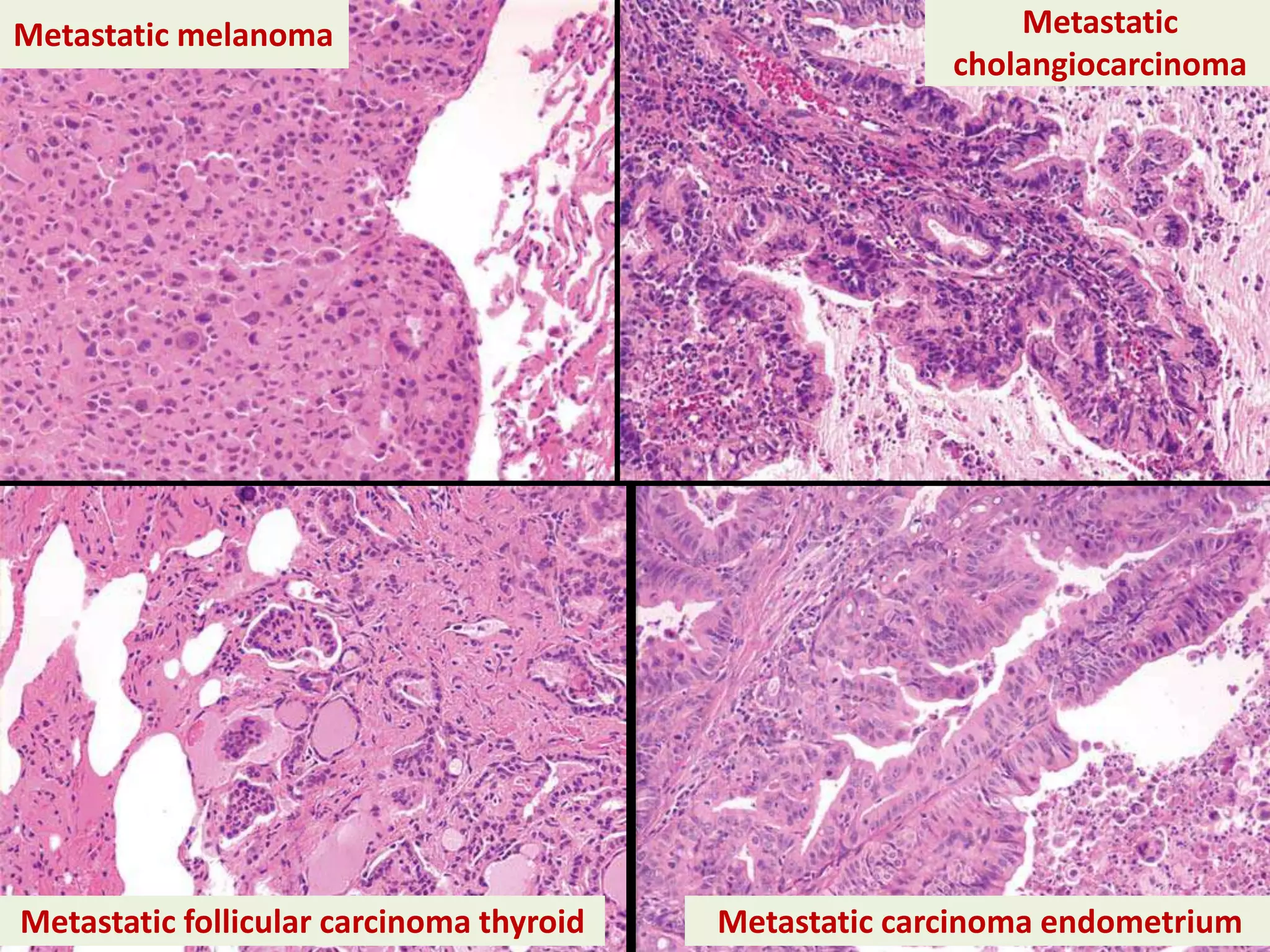
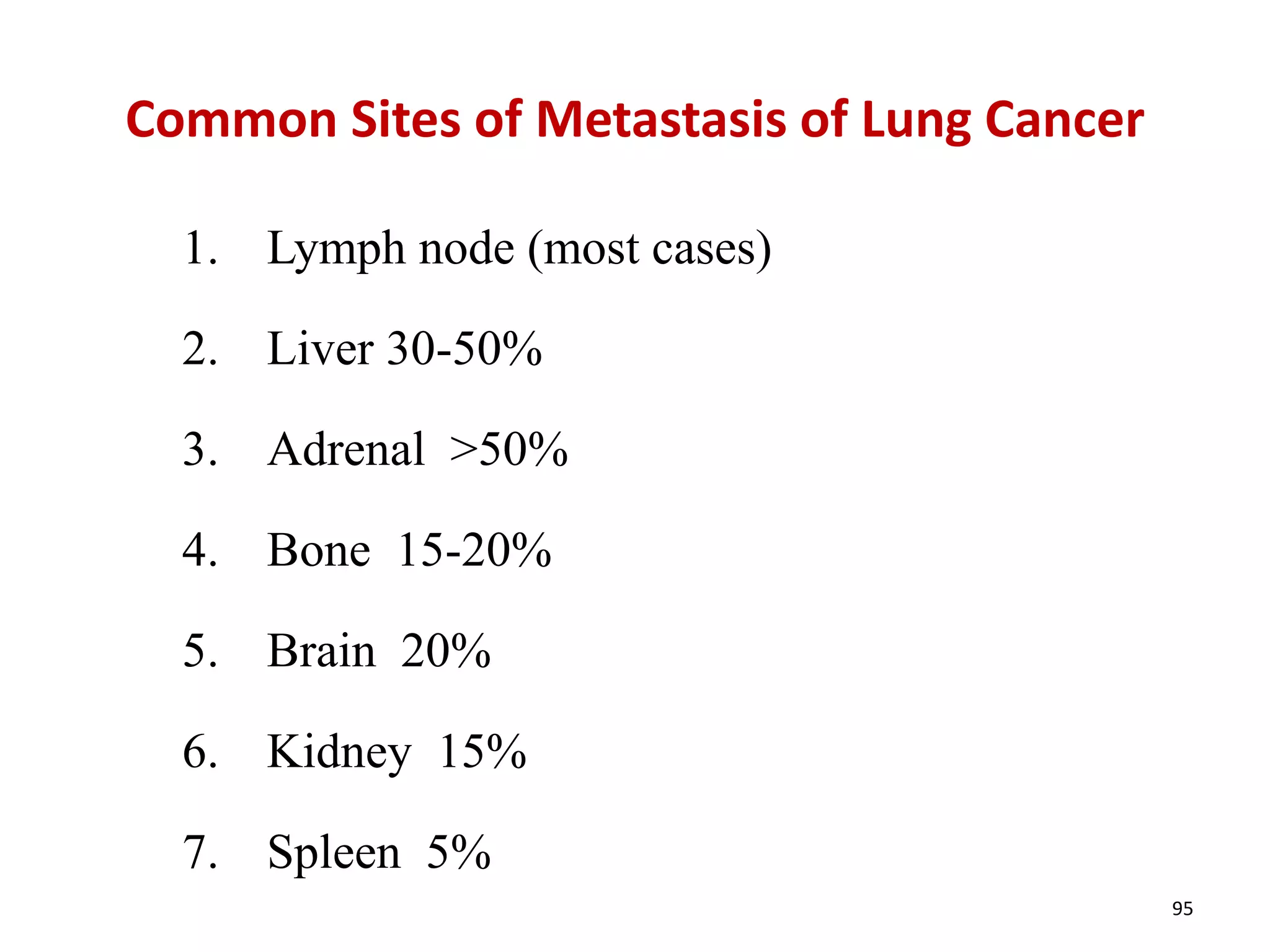
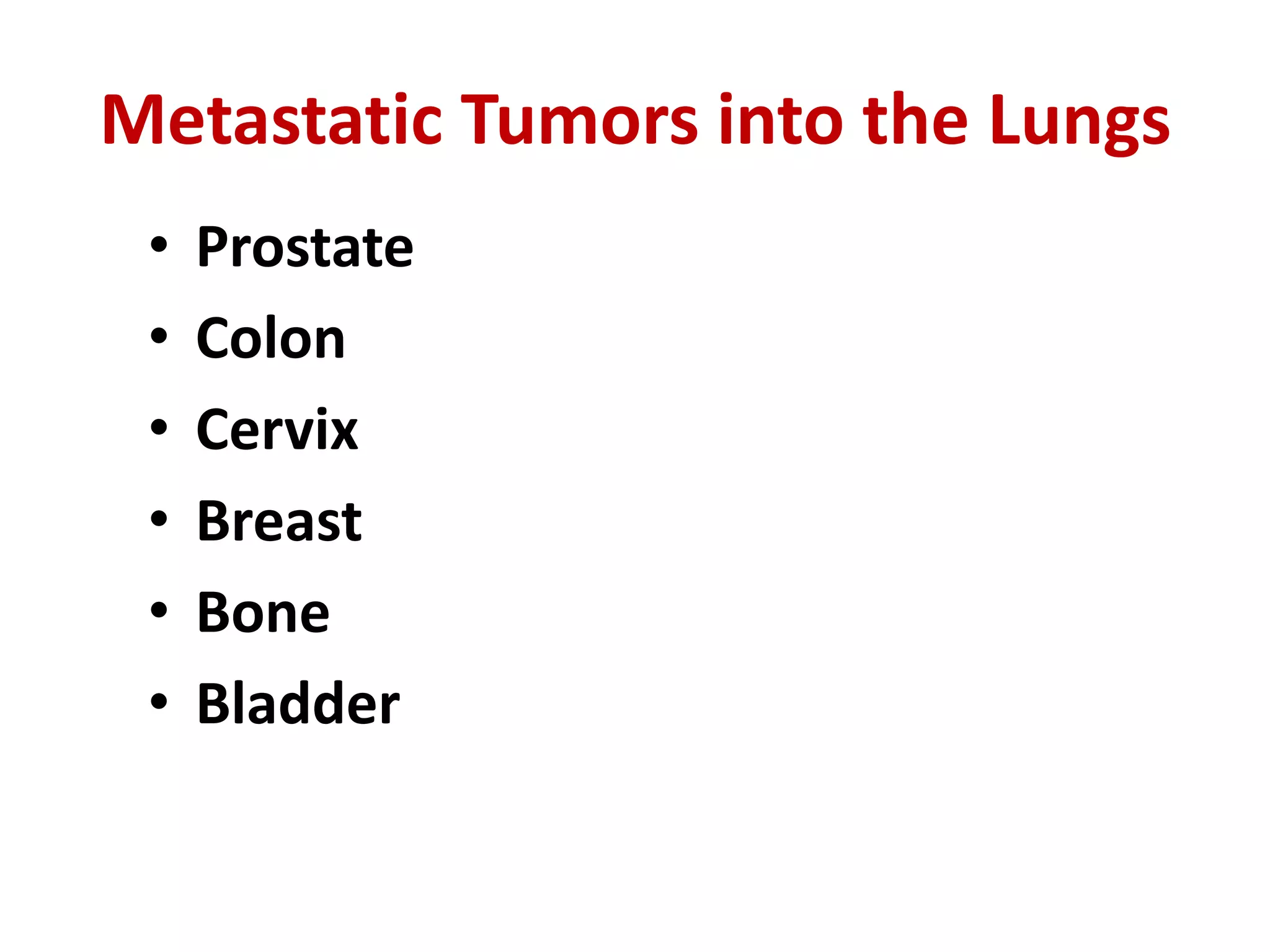
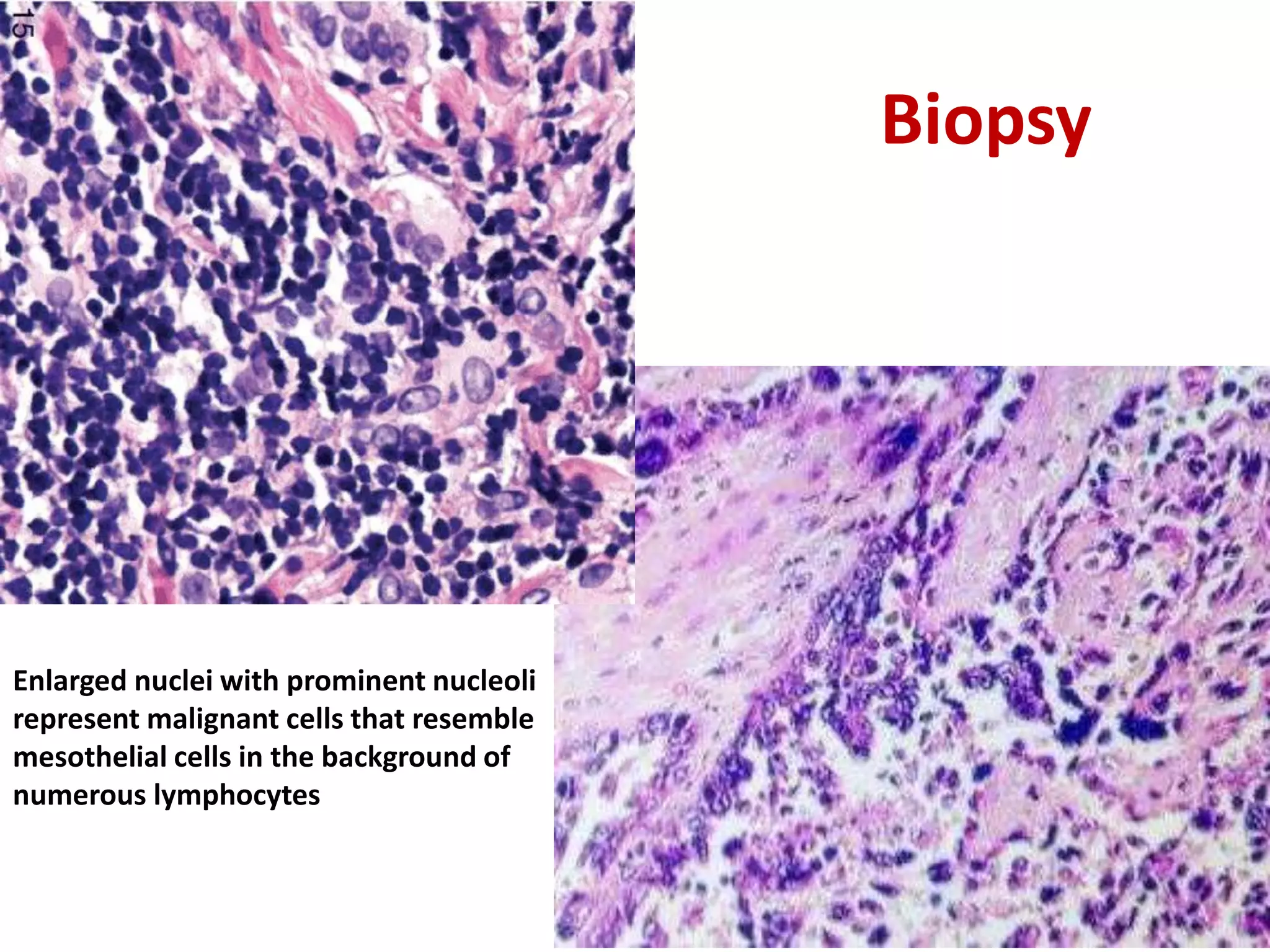
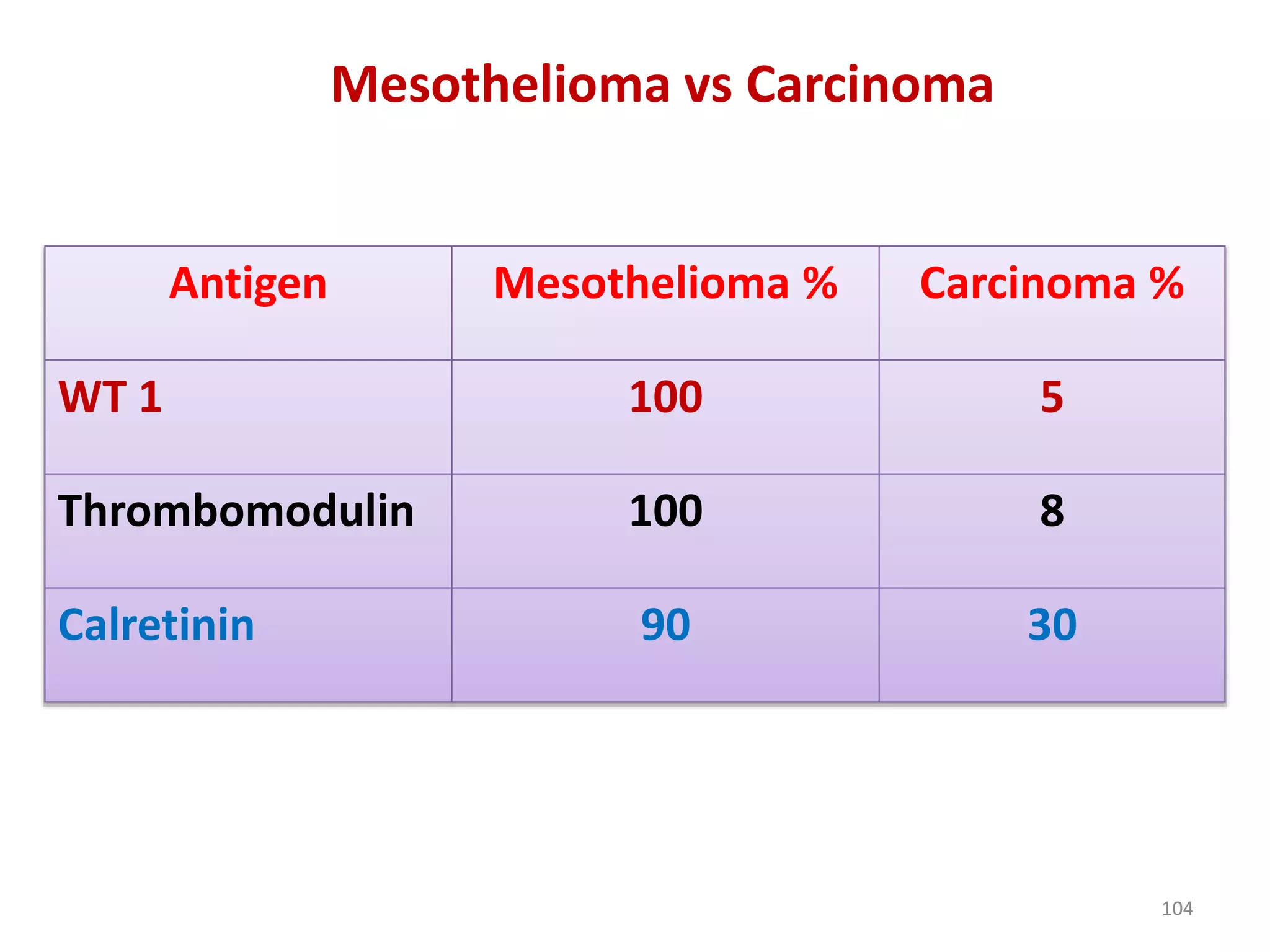
The document provides a comprehensive classification of lung tumors, including primary and metastatic types, and details various types of epithelial tumors, mesenchymal tumors, and lymphohistiocytic tumors. It elaborates on specific subtypes, their characteristics, diagnostic criteria, and treatment approaches, particularly emphasizing the distinctions between small cell and non-small cell lung cancers. The document also discusses histological features, imaging findings, and immunohistochemical markers relevant to lung cancer diagnosis.