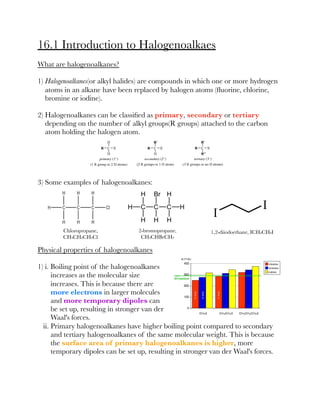
This document summarizes key information about halogenoalkanes:
- Halogenoalkanes undergo nucleophilic substitution reactions like hydrolysis and reactions with cyanide ions or ammonia via SN1 or SN2 mechanisms. Tertiary halogenoalkanes favor SN1 while primary favor SN2.
- Chlorofluorocarbons (CFCs) were widely used but deplete the ozone layer. Their alternatives like HCFCs are less damaging but still pose issues.
- Other uses of halogenoalkanes include their inertness making fluoroalkanes useful as refrigerants and propellants, though concerns about ozone depletion drove a search for replacements like propane