Embed presentation
Download as PDF, PPTX

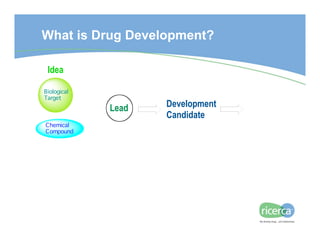
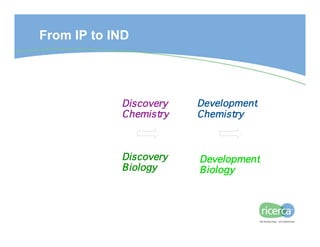



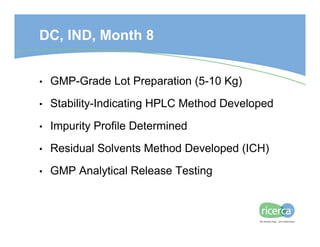

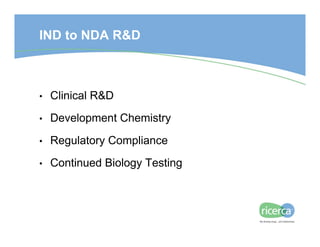

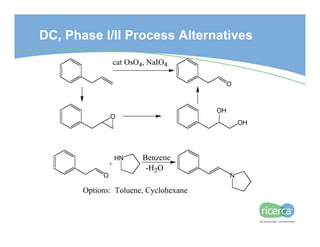

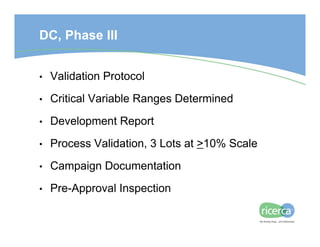

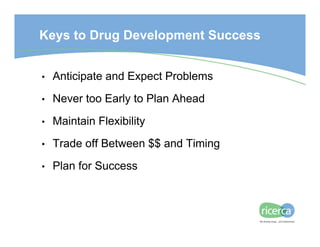


The document outlines the drug development process, focusing on the transition from a lead candidate to an Investigational New Drug (IND) application. It details key phases including chemistry development, toxicology, regulatory compliance, and the importance of planning for potential issues. Strategies for successful development emphasize flexibility, anticipation of problems, and careful management of timelines and costs.


















