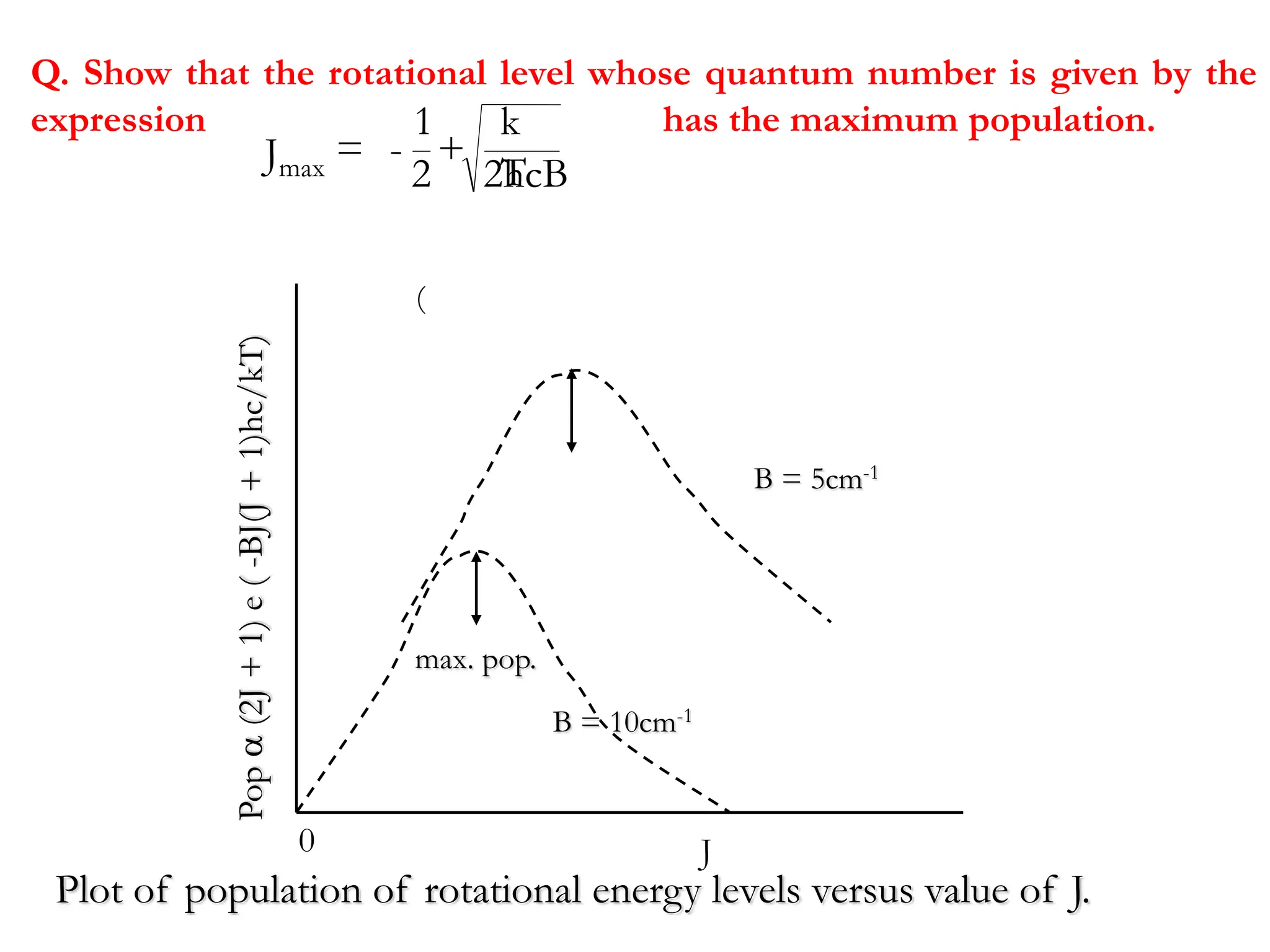
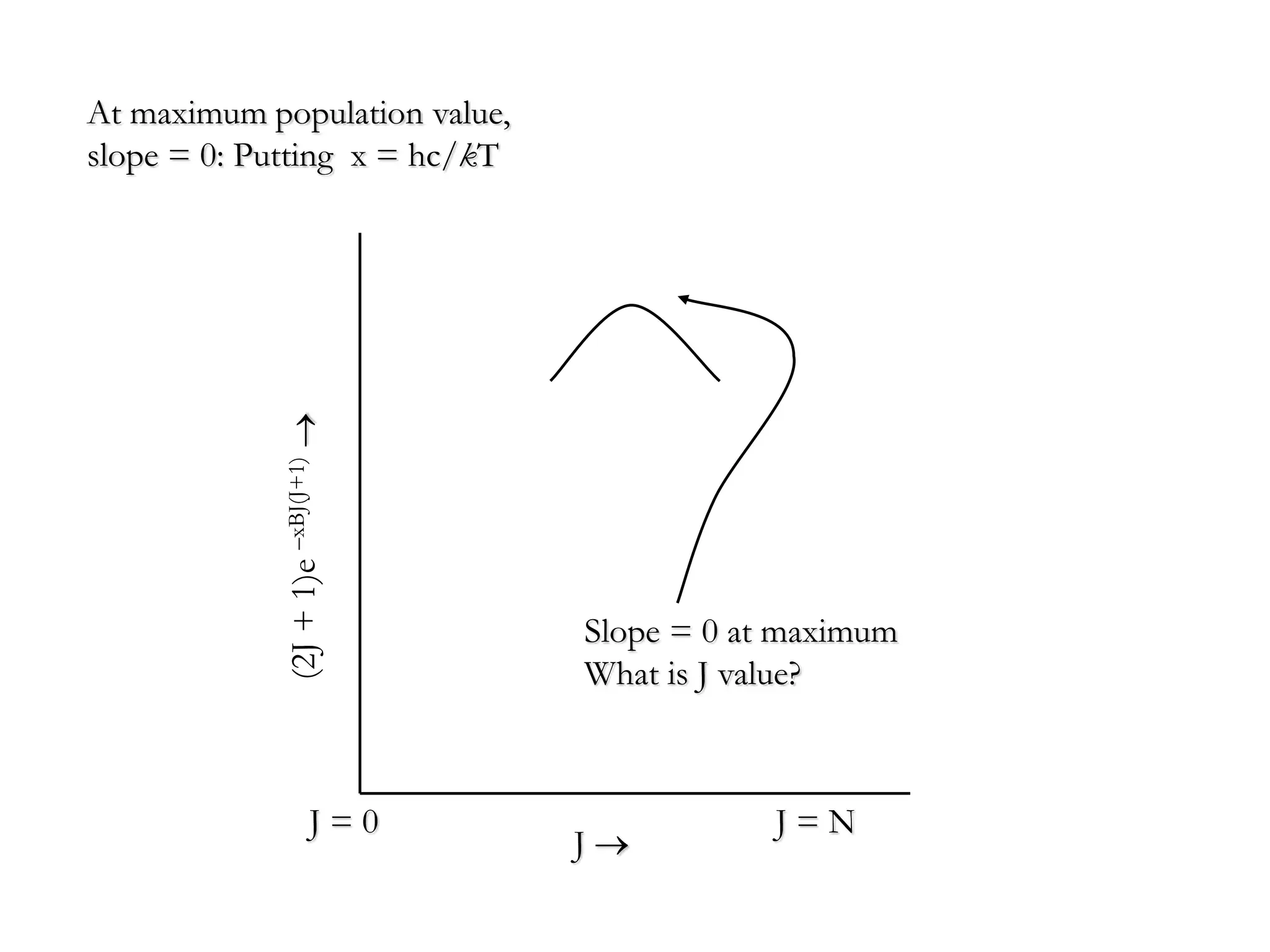
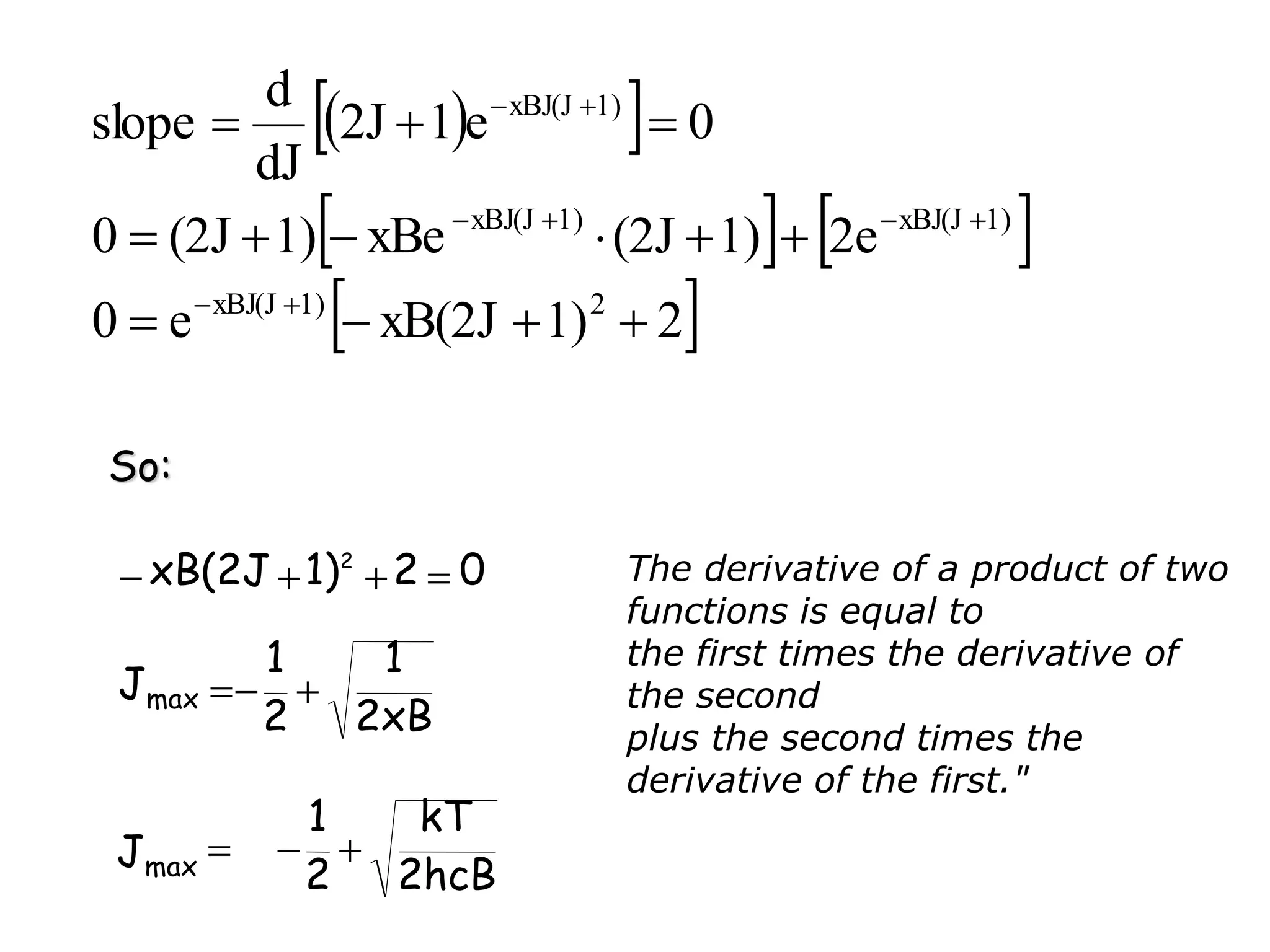
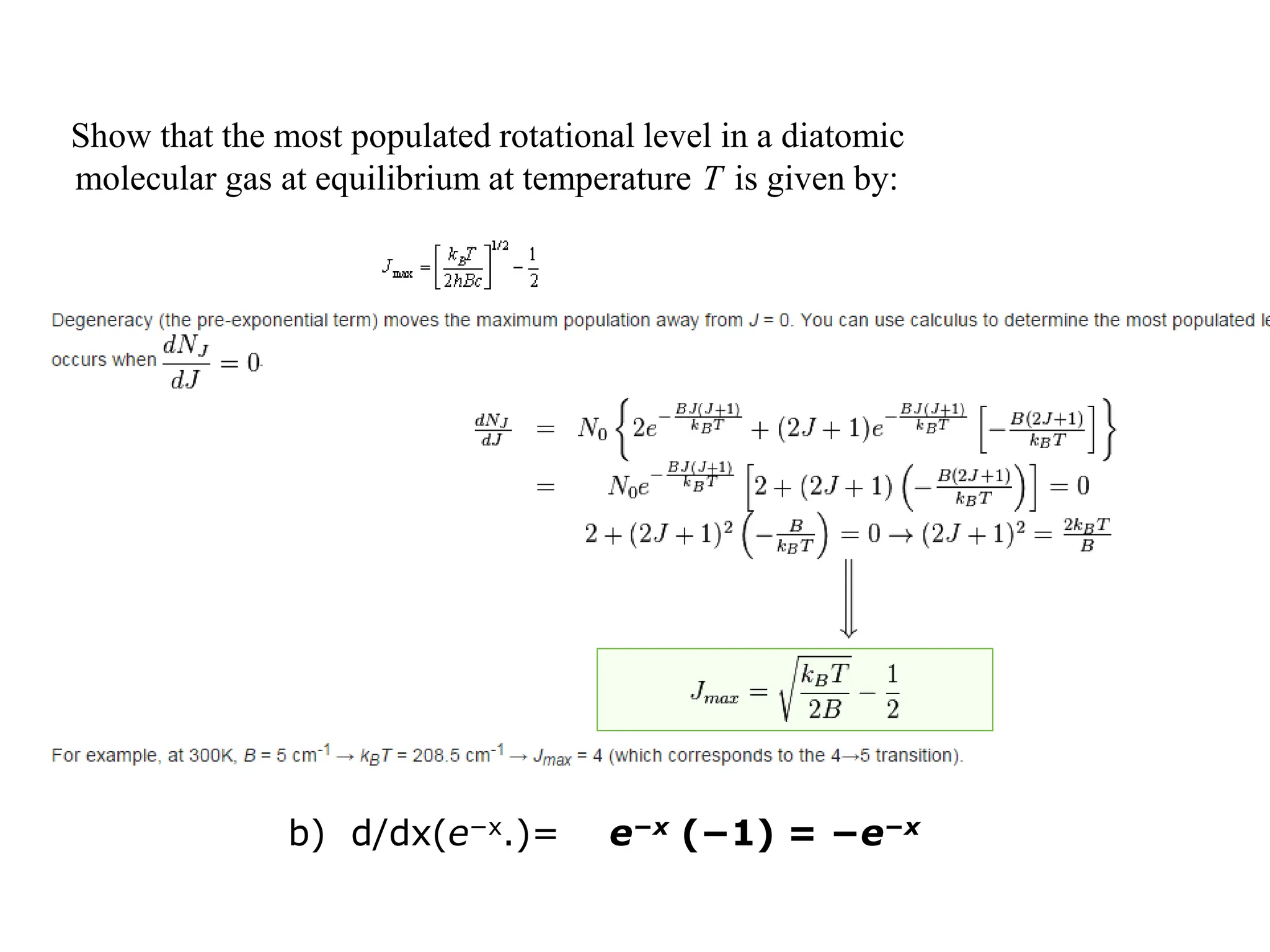
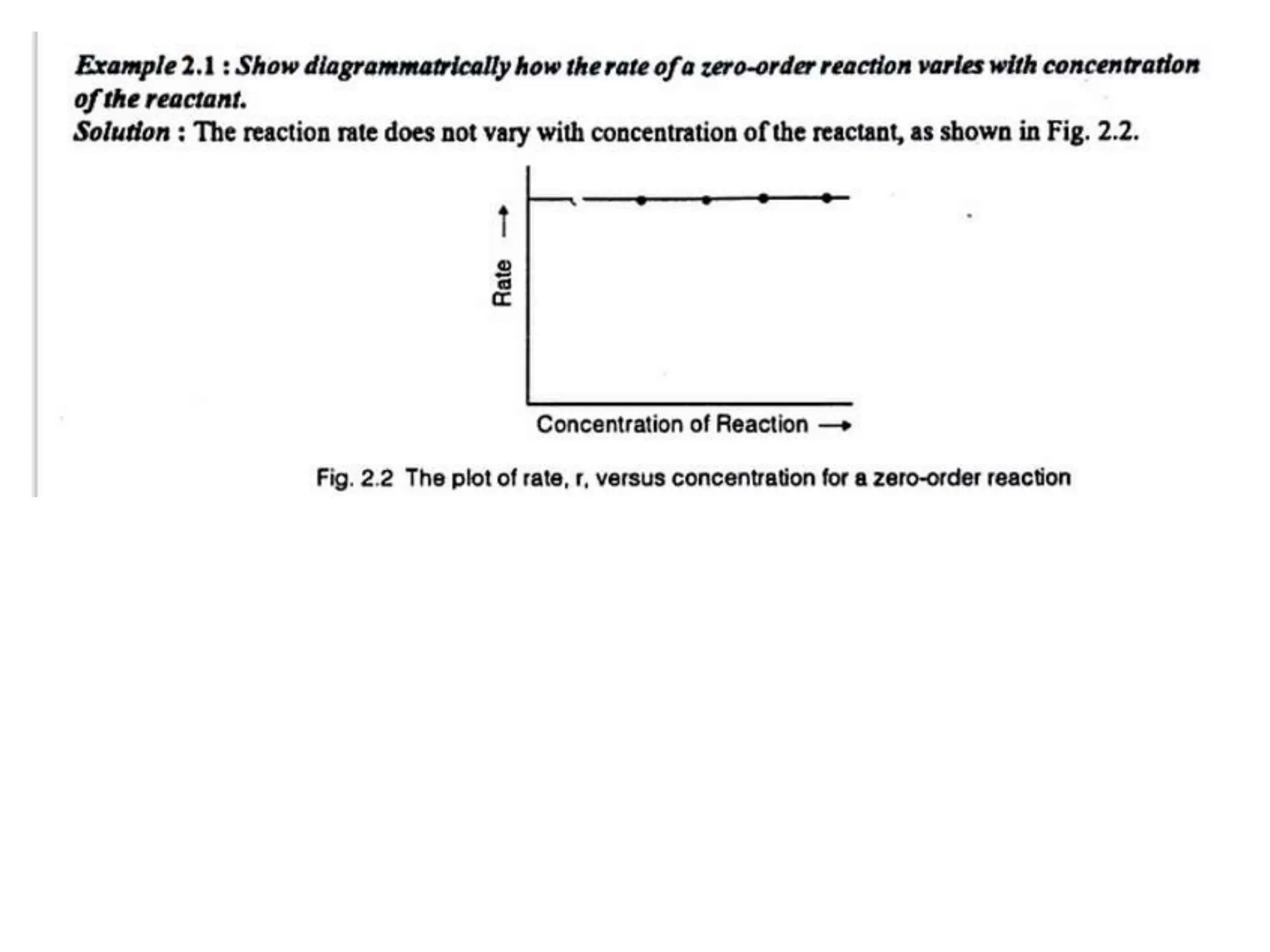
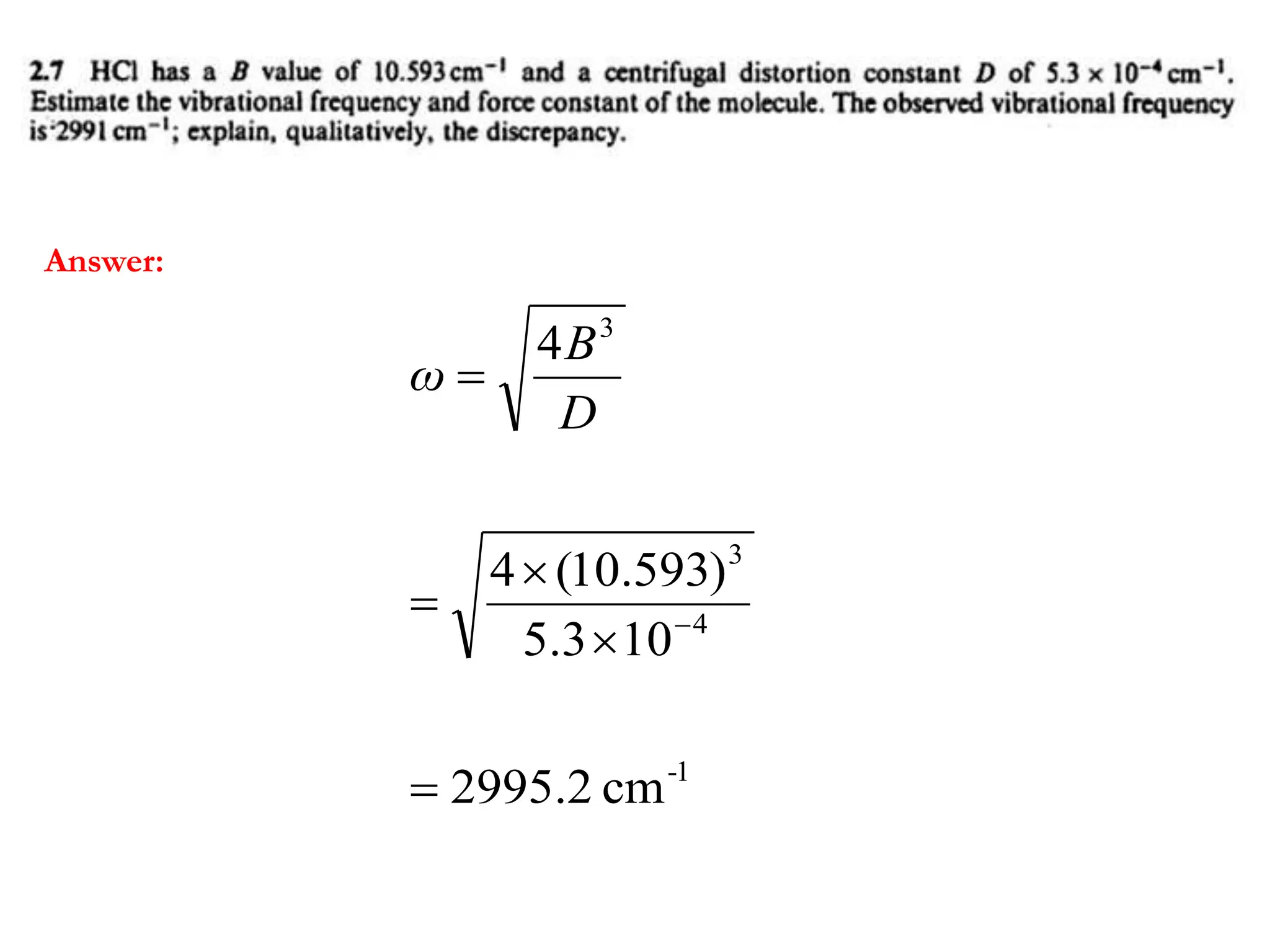
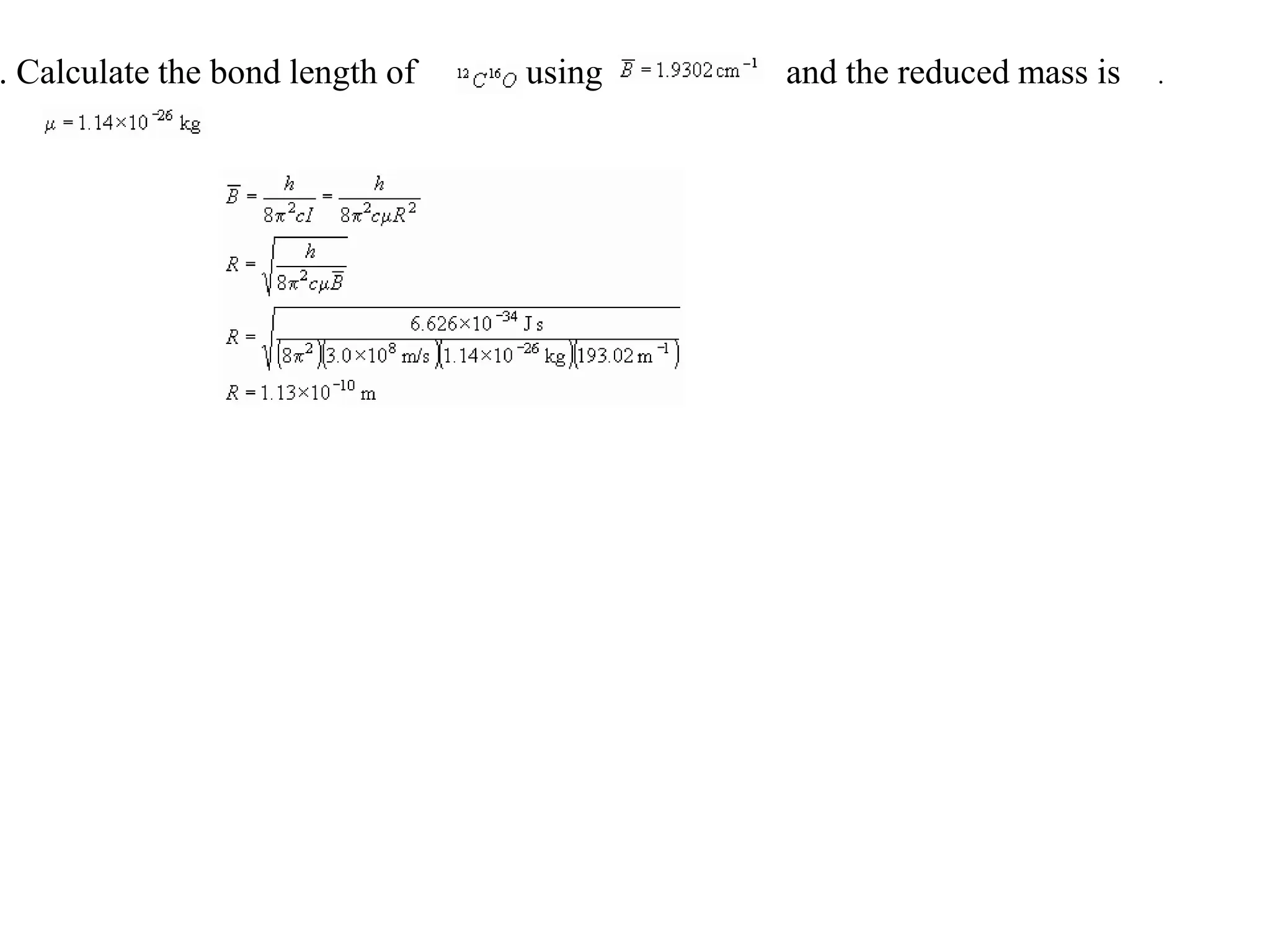
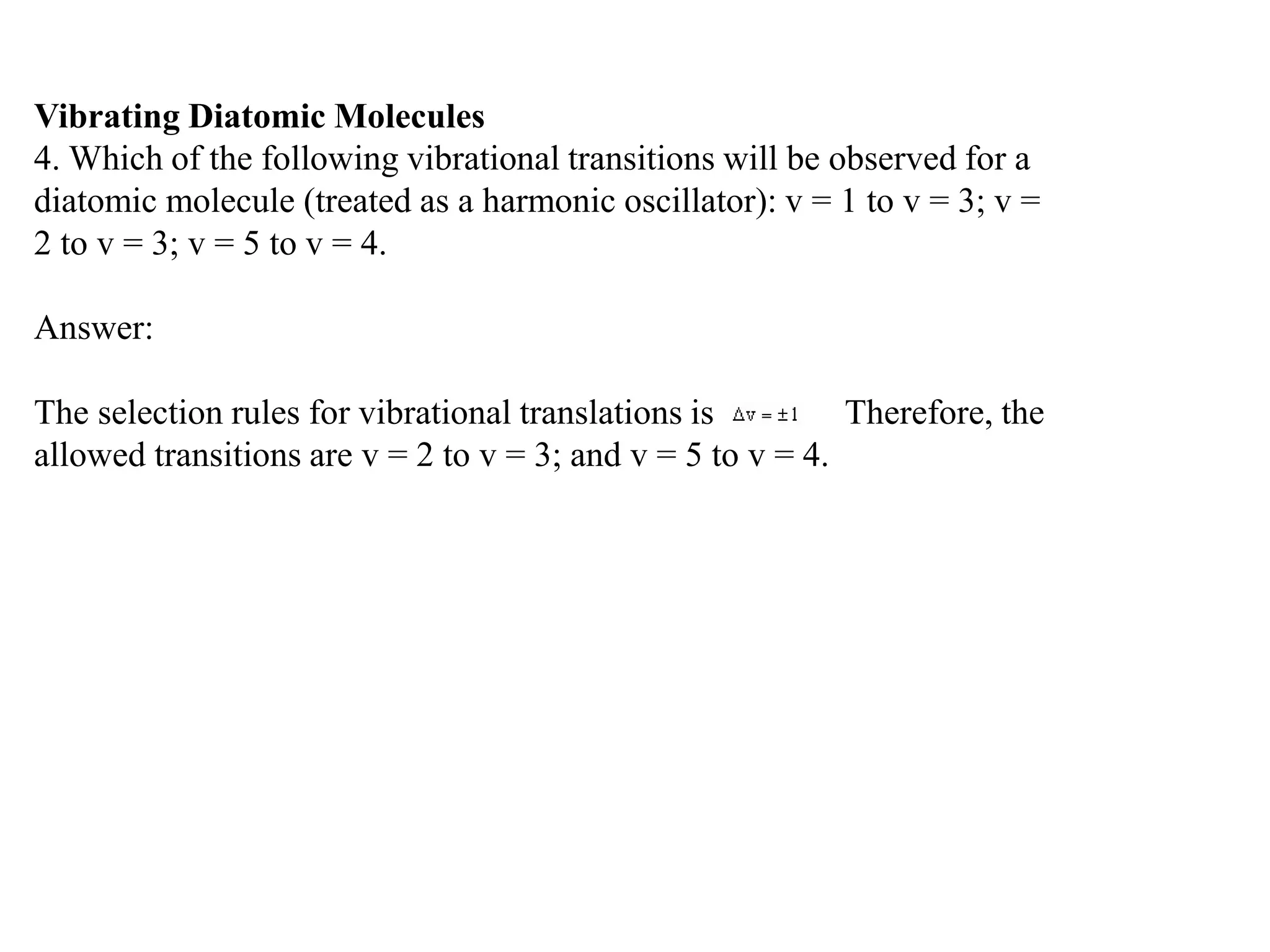
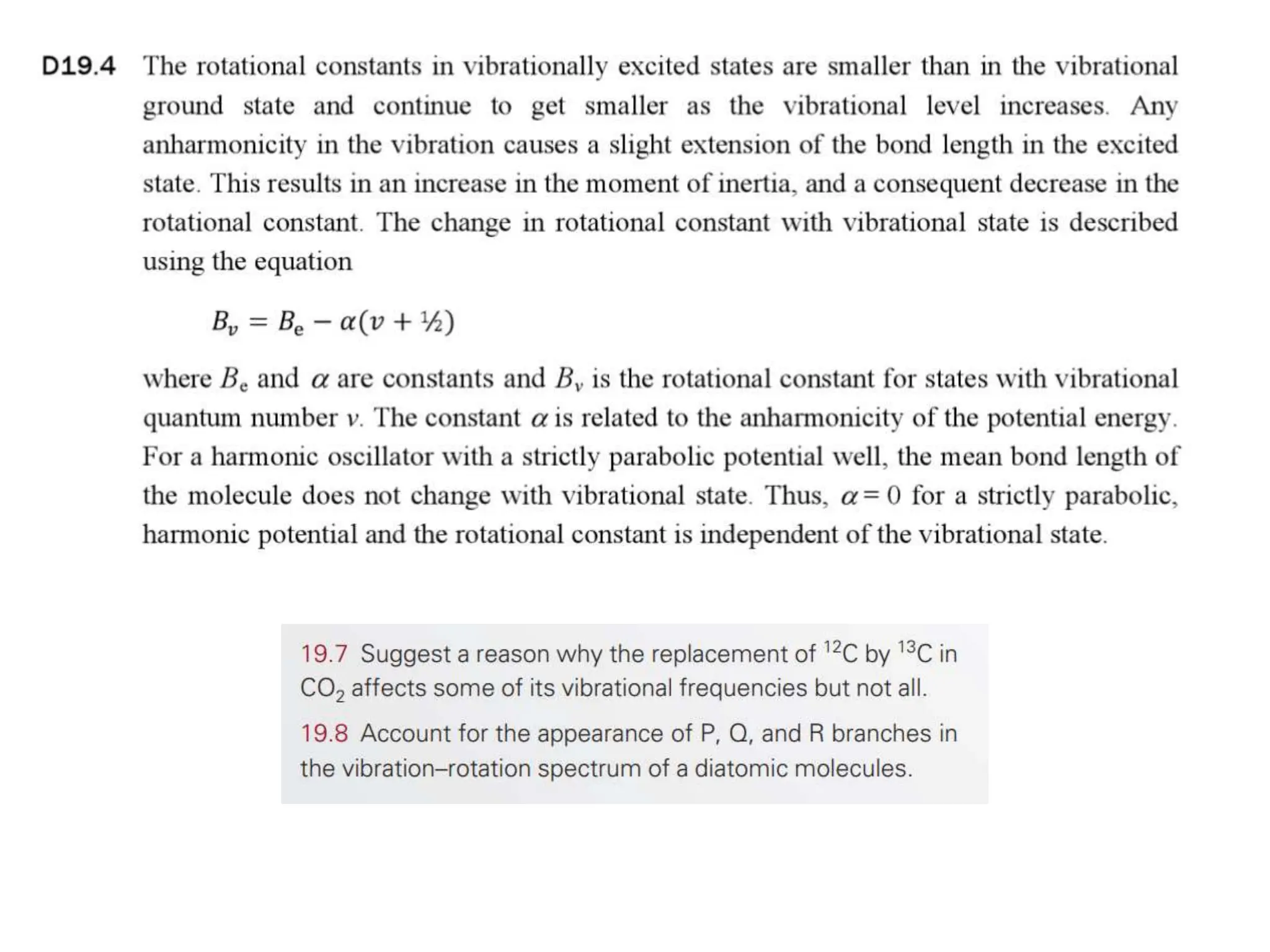
The document discusses the population of rotational energy levels in diatomic molecular gases, deriving expressions for maximum population and the conditions for equilibrium at temperature T. It also analyzes the molecular shapes of certain compounds and provides calculation details pertinent to bond lengths and vibrational transitions in harmonic oscillators. The allowed vibrational transitions based on selection rules are identified.