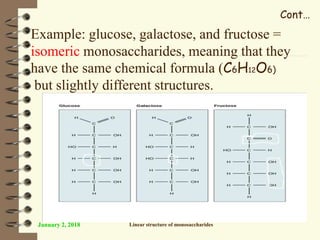
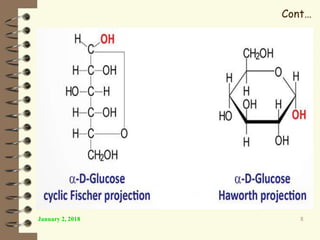
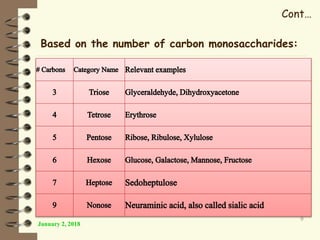
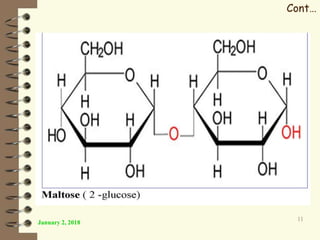
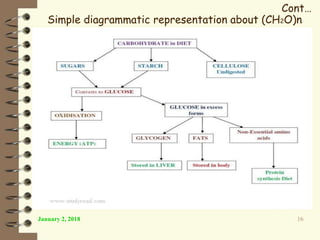
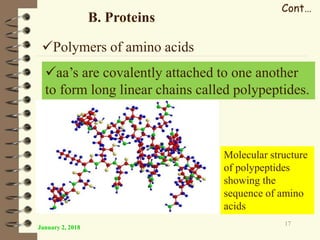
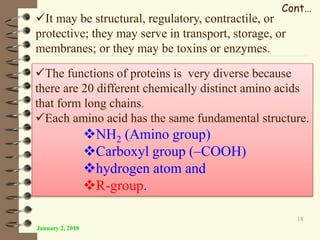
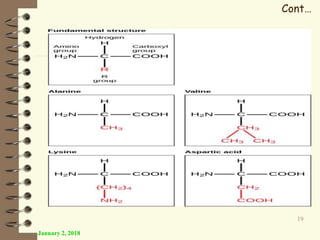
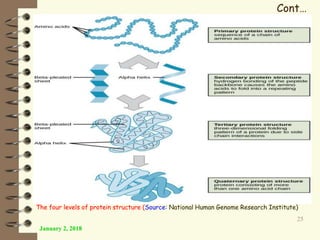
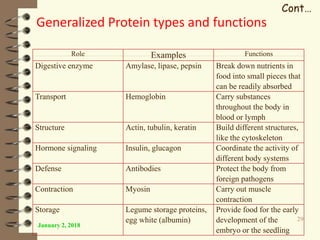
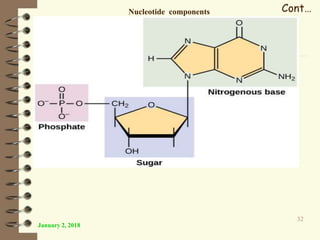
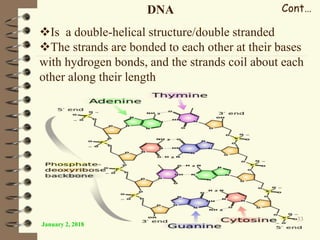
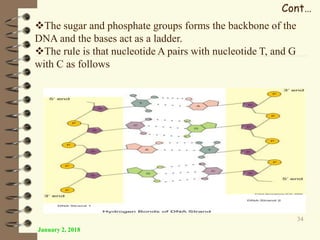
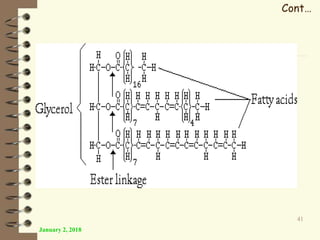
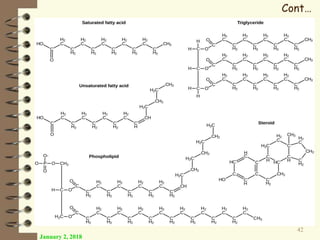
This document provides an outline and introduction to a lecture on biochemical and physiological uses of macromolecules. It discusses the four main types of macromolecules - carbohydrates, proteins, nucleic acids, and lipids. For each macromolecule, it describes their monomer units, structures, functions, and roles in biochemical processes and physiology. The document is intended to give postgraduate students an overview of the key topics that will be covered in the upcoming lecture.