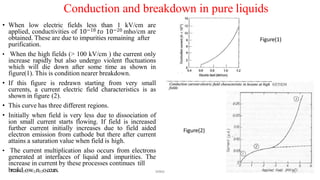
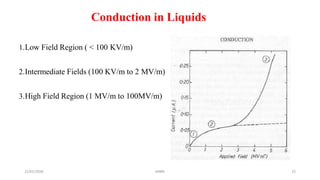
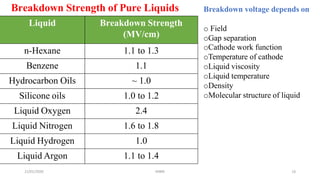
The document discusses various theories related to breakdown in liquid dielectrics. It begins with an introduction to pure and commercial liquids, and different breakdown theories. Some of the key theories discussed include the suspended particle theory, cavitation and bubble theory, and stressed oil volume theory. The document also covers factors that affect breakdown strength such as impurities, gas content, liquid viscosity, and stressed volume. Thermal breakdown mechanisms are discussed as well. A variety of liquid dielectric materials and their typical breakdown strengths are also listed.











![Suspended particle theory
Reasons responsible for breakdown
• In commercial liquids, solid impurities will be present as fibres or as dispersed solid particles.
• If ∈2 is the permittivity of solid particles & ∈1 is the permittivity of liquid.
• If we consider these impurities to be spherical particles of radius r, and applied field is E, then the particle
experience force F, where
F= ½ 𝑟3 [(∈2 - ∈1) / (2∈1+∈2)]*Grad𝐸2
• If ∈2 > ∈1, e. g. In case of presence of solid particles like paper in liquid force is directed towards
maximum stress.
• The force will be in direction of areas of lower stress if only gas bubbles are present in liquid i.e. ∈2 < ∈1.
• This force drives the particle towards areas of maximum stress if voltage is continuously applied (D.C.) or
the duration of voltage is long (A.C.)
• Thus a stable chain of suspended particles can form which may bridge electrode gap if number of particles
are more. This will lead to breakdown.
• In case of less number of suspended particles or these may be single conducting particle between
electrodes, it will rise to local field enhancement depending on its shape.
• Local breakdown occurs near particle when field exceeds breakdown voltage of liquid.
• Th21u/0s1/g20a2s0bubbles are formed which may lead to breMaBkMdownof liquid. 18](https://image.slidesharecdn.com/chapter2breakdowninliquids-200316135736/85/Chapter-2-breakdown-in-liquids-18-320.jpg)

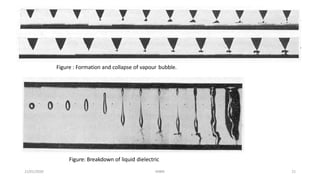
![Cavitation and bubble theory
• According to this theory a kind of vapour bubble formed is responsible for breakdown. Vapour bubbles may
form in liquids due to following reasons.
1. Between space charges electrostatic repulsive forces may exist and they may be sufficient to overcome
surface tension.
2. Electron collisions cause dissociations of liquid molecules and thus gases are produced.
3. Gas pockets may be available at surface of electrode.
4. Sharp points and irregularities on electrode surface causes vaporization of liquid by corona type discharge.
• Electrostatic forces will act on bubble and it will elongate in direction of electric field.
• When the voltage drop along the length of bubble becomes equal to minimum value on Paschens curve for
gas in bubble breakdown occurs.
𝐸0 = 1/(∈1 - ∈2) [ 2𝜋𝜎 ( 2 ∈1 + ∈2 ) /r { 𝜋/4 𝑉 𝑏/2𝑟𝐸0 - 1}]^ ½
Where,
21/01/2020 MBM 20
𝜎- surface tension of liquid.
𝑉𝑏- Voltage drop in bubble.
∈1& ∈2 - Permittivity of liquid & gas bubble respectively
r – initial radius of bubble as sphere.](https://image.slidesharecdn.com/chapter2breakdowninliquids-200316135736/85/Chapter-2-breakdown-in-liquids-20-320.jpg)