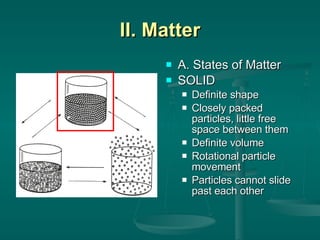
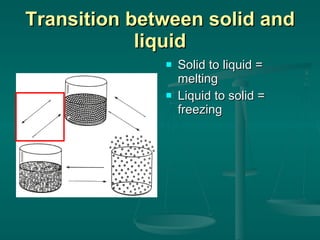
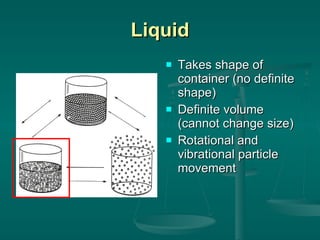
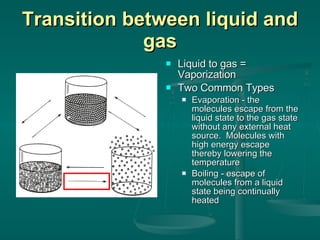
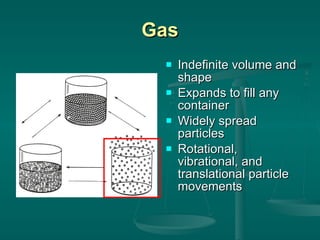
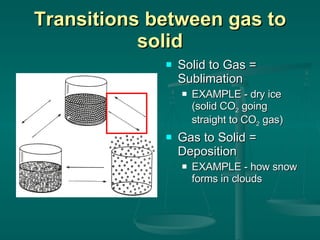
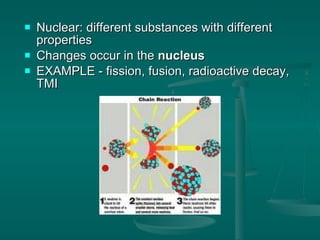
This document provides an introduction to chemistry and matter. It discusses that chemistry is the study of matter and its properties. There are several branches of chemistry including organic, inorganic, physical, analytical, biochemistry, and theoretical chemistry. Matter can exist in solid, liquid, or gas states depending on the closeness and movement of its particles. A substance's properties, whether physical or chemical, can help identify it. Changes to matter can be physical, chemical, or nuclear, altering its identity or structure on different levels, while conserving total mass and energy.