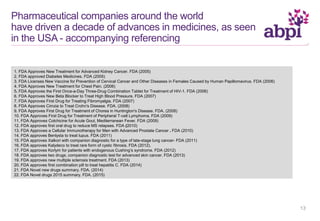
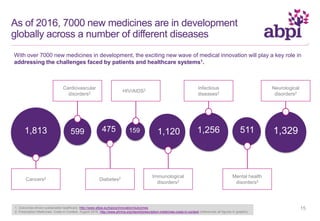
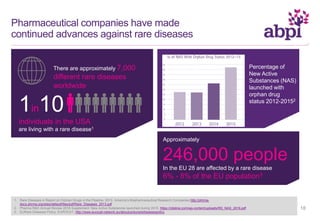
The document discusses the role of pharmaceutical companies in advancing medicine and healthcare through innovative treatments and vaccinations, leading to improved patient outcomes globally. It highlights the significant progress made in drug development over the past decade, including numerous approvals for various diseases and conditions. Additionally, it emphasizes the importance of academic and industry partnerships in facilitating medical research and development.