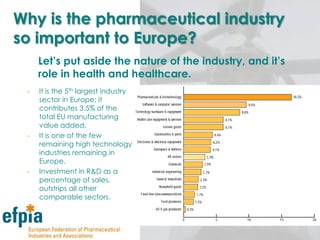
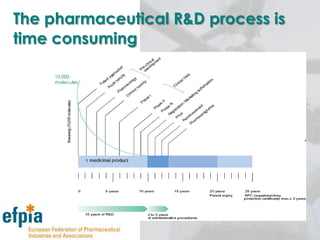
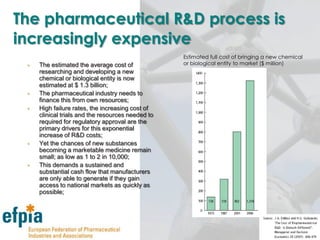
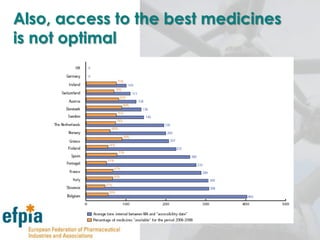
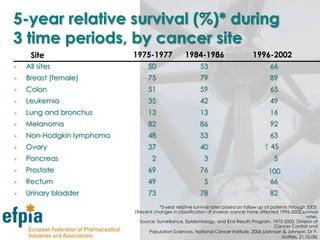
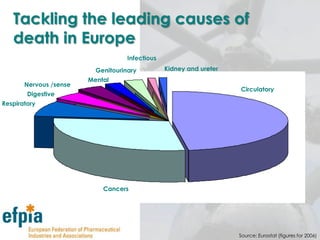
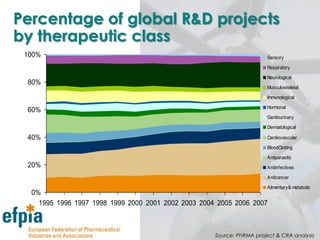
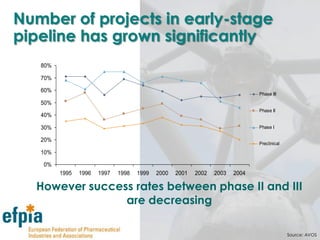
The document discusses the importance of the pharmaceutical industry to Europe. It summarizes that the industry directly employs over 630,000 people in Europe and contributes 3.5% of EU manufacturing value. It is one of the few remaining high-technology industries in Europe. The industry also plays a dual role as a major economic contributor and in improving health outcomes for European citizens. However, access to new medicines remains a challenge due to the high costs of research and regulatory hurdles.