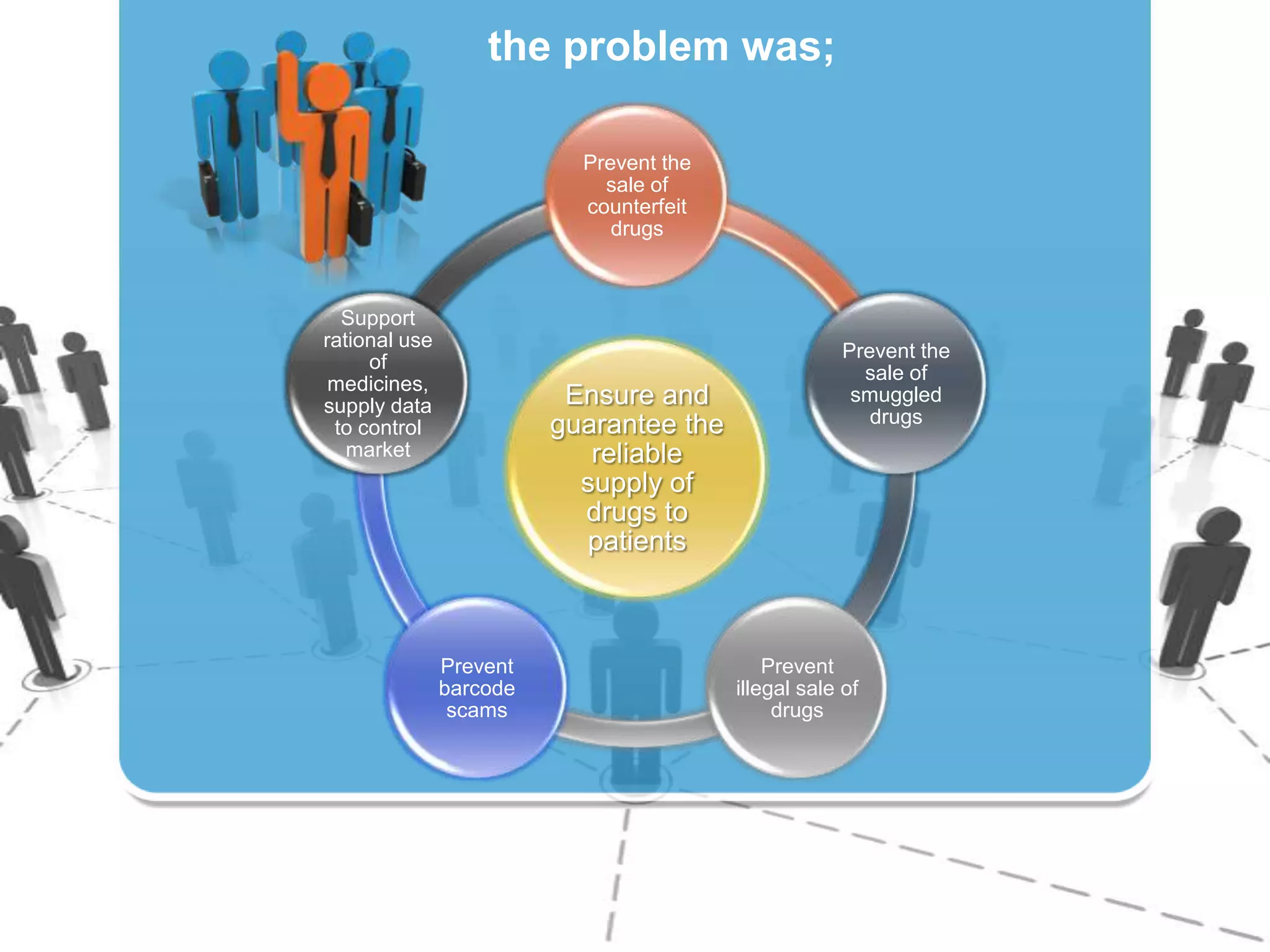
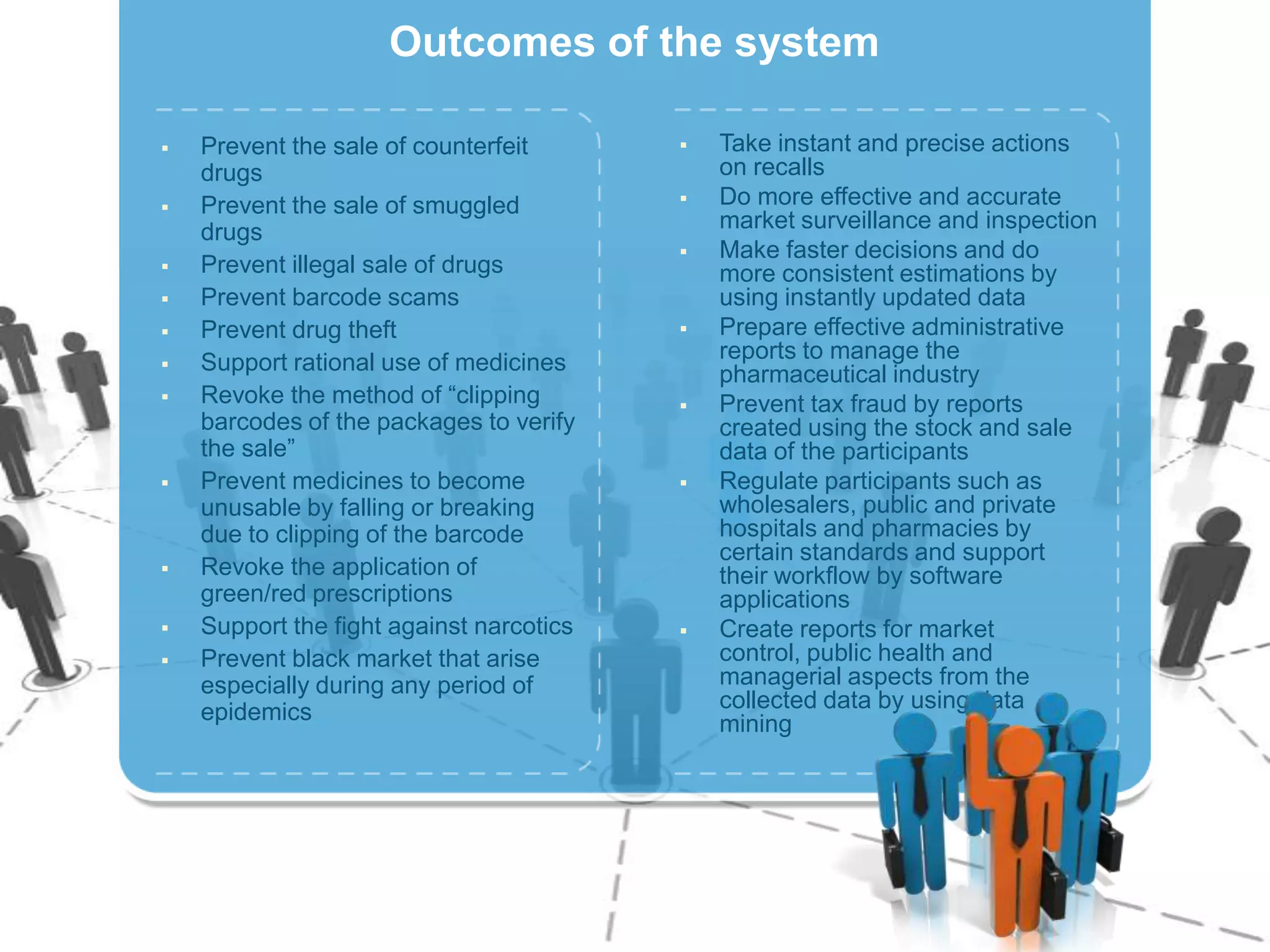
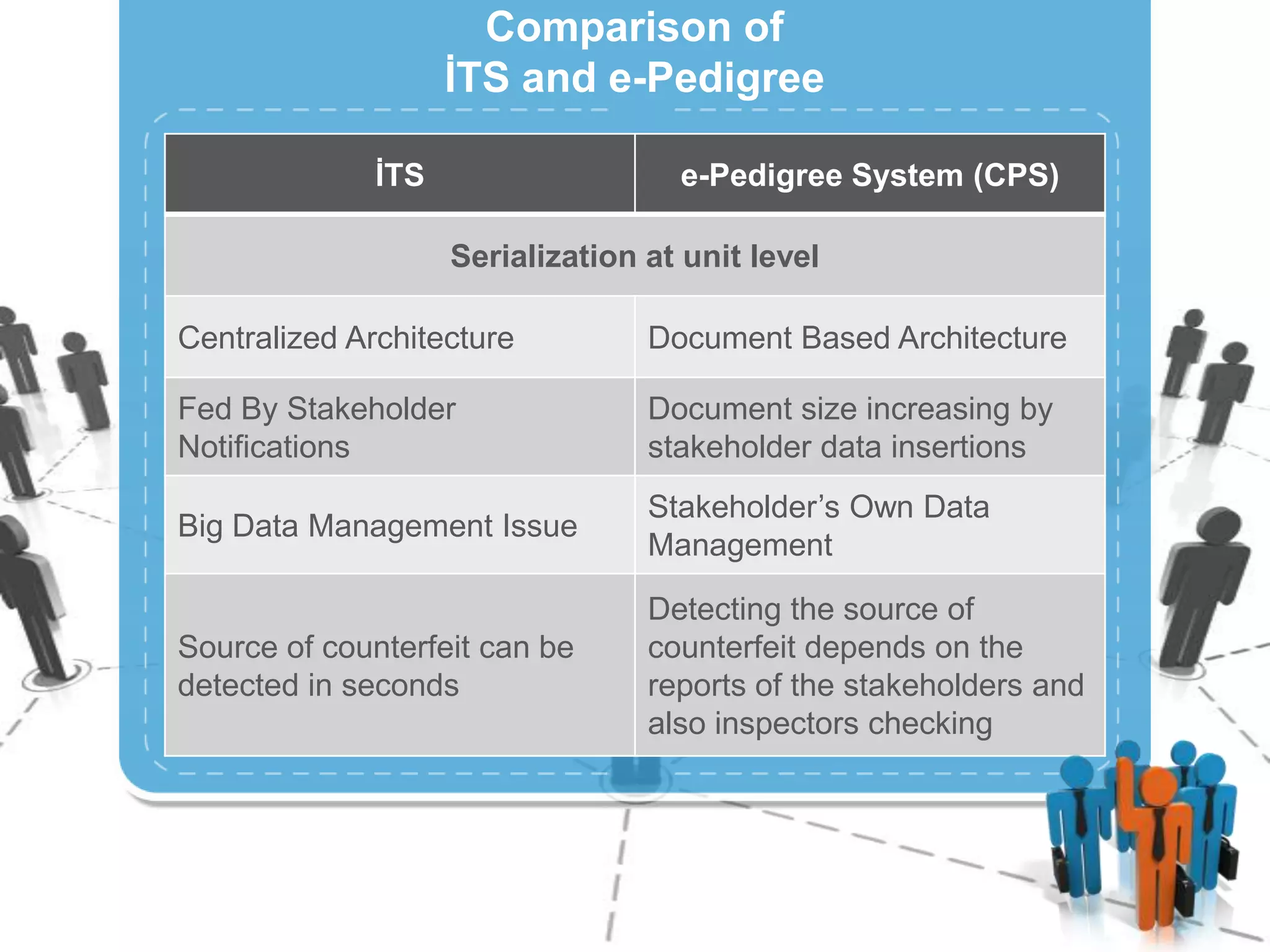
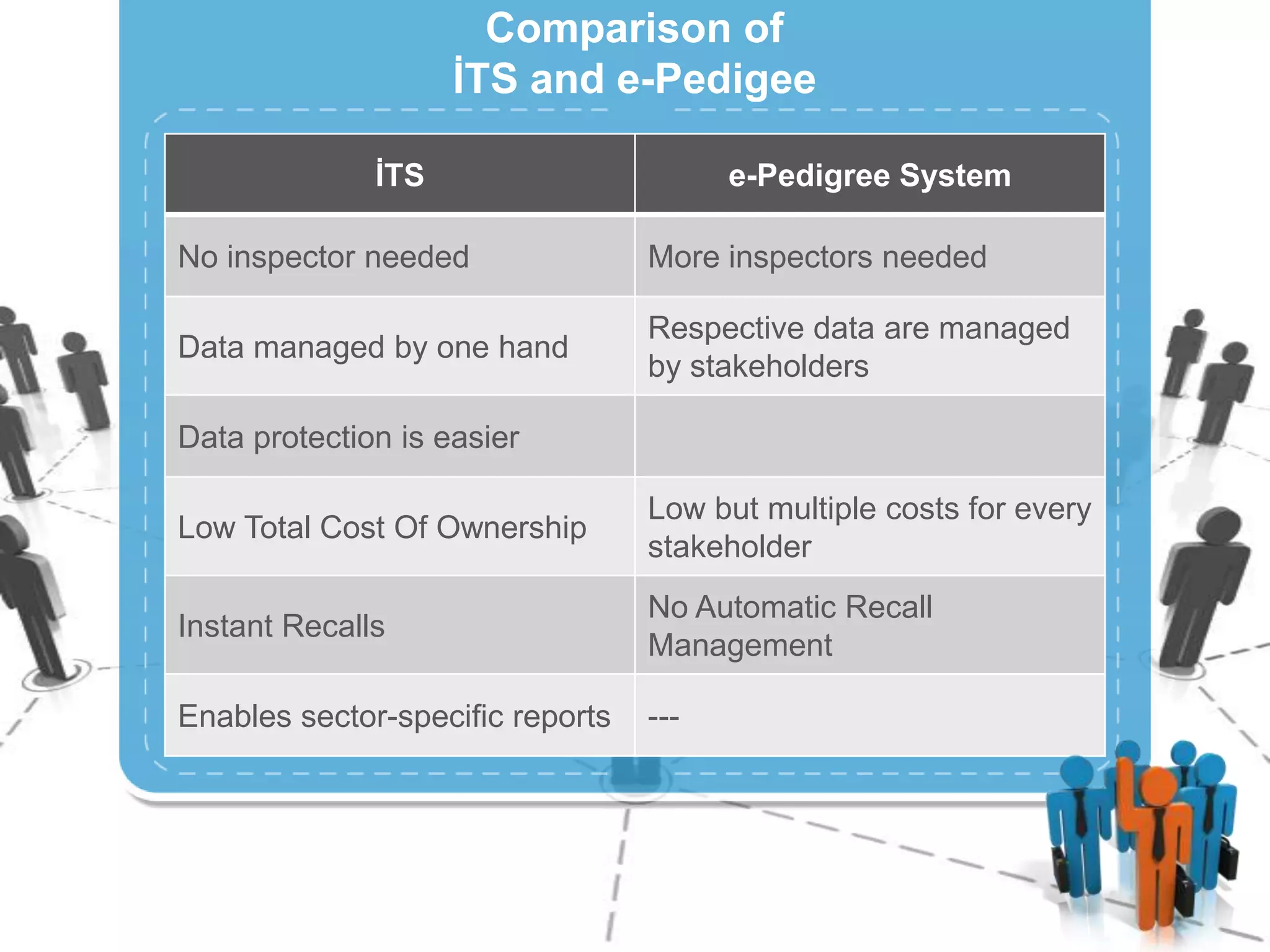
The document describes Turkey's Pharmaceutical Track and Trace System (ITS) which was implemented to address issues like counterfeit drugs and drug theft. The key aspects of the system include:
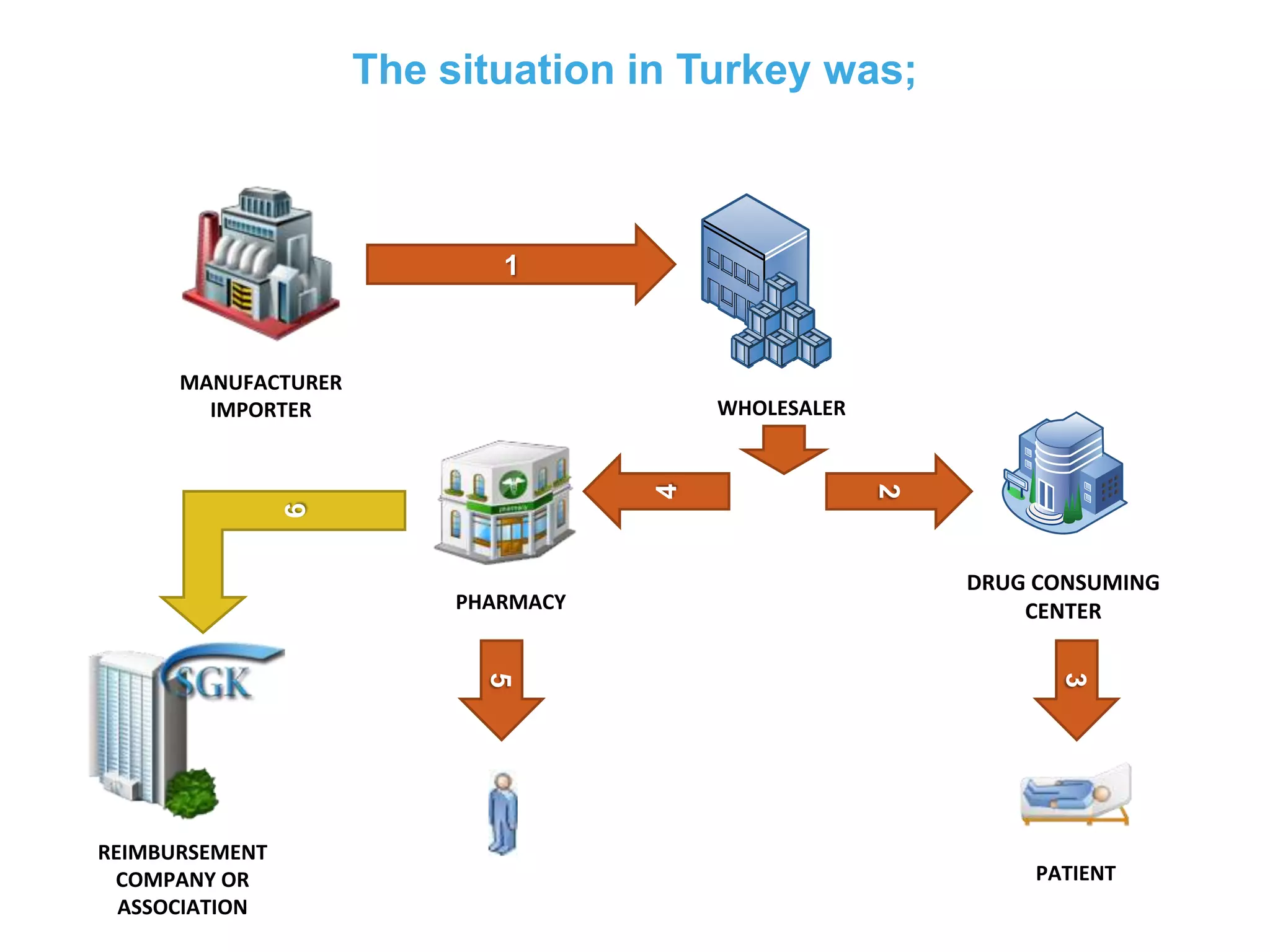
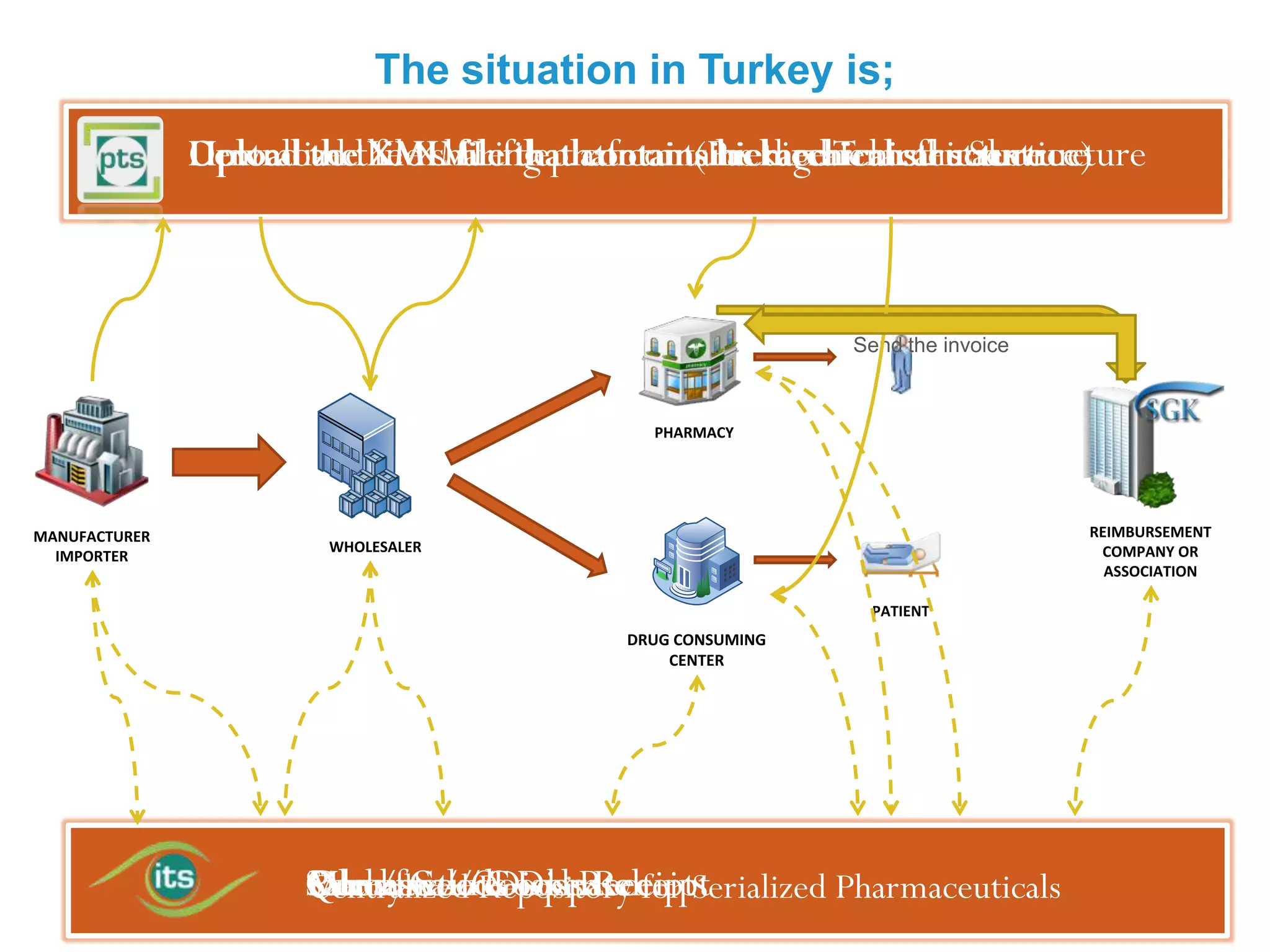
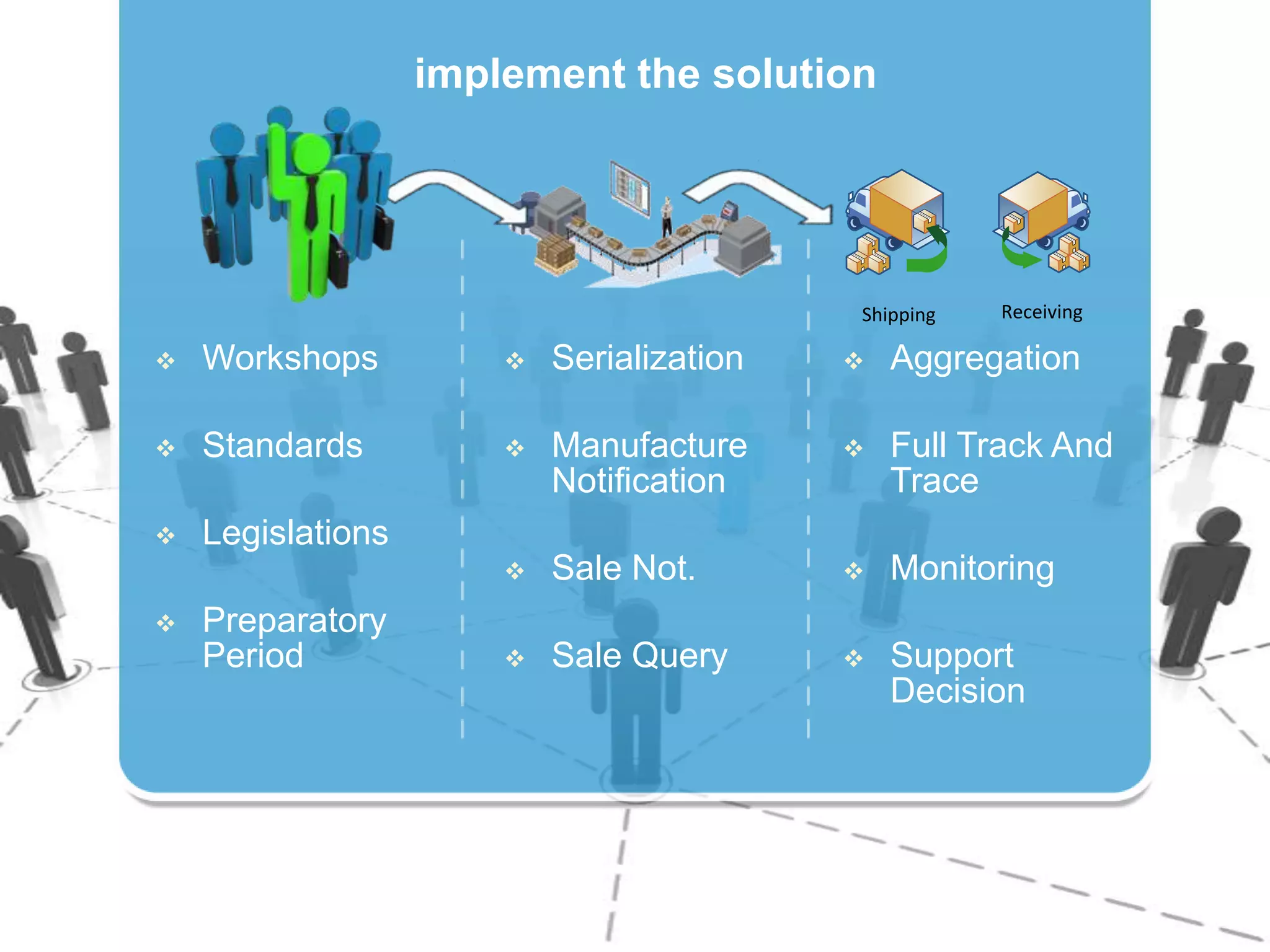
1) Serializing each drug unit with a unique DataMatrix code for tracking through the supply chain from manufacturer to patient.
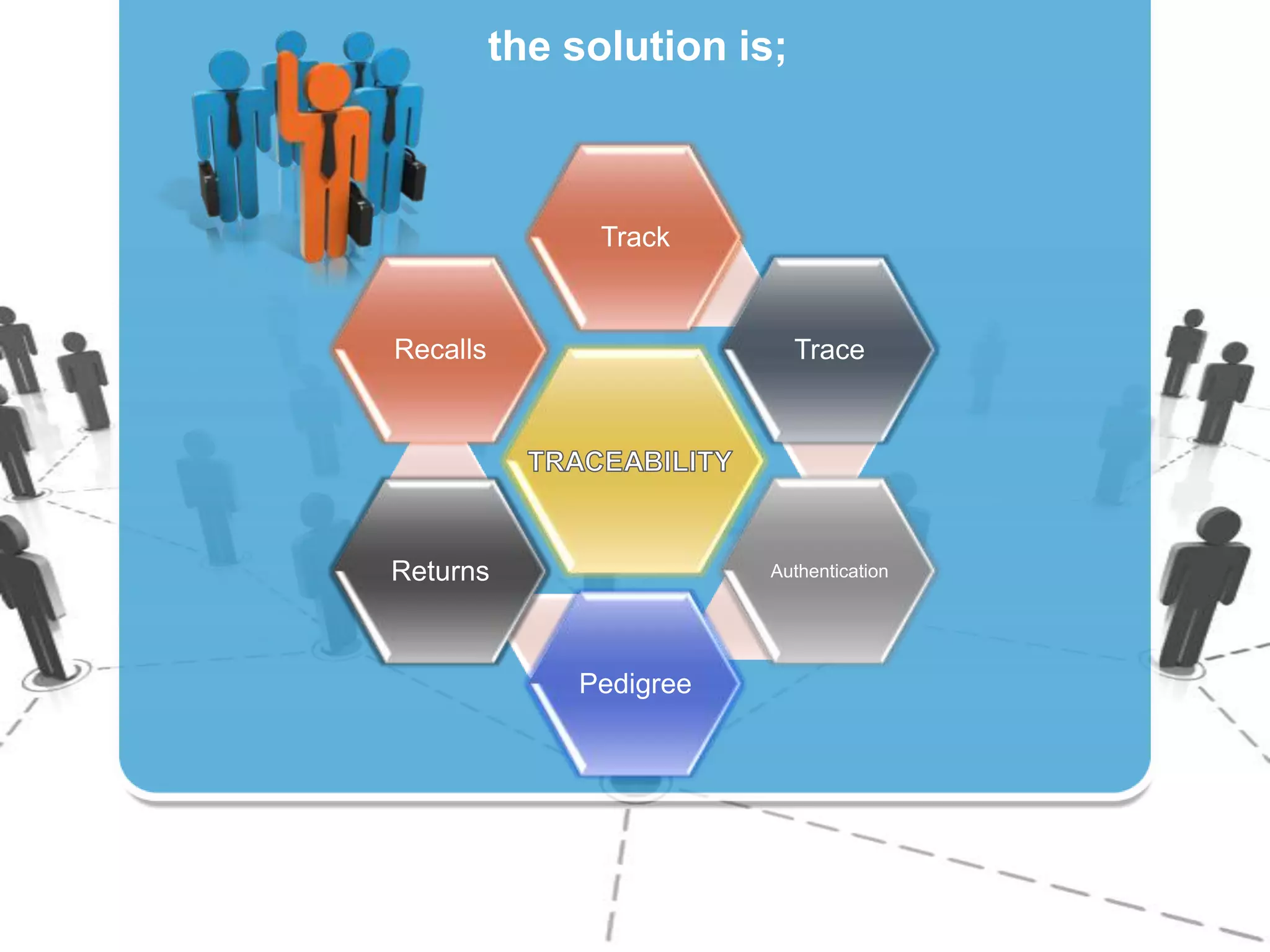
2) Gathering data on each drug unit at each step to enable full tracking and traceability through electronic pedigrees.
3) Centralizing the system for more efficient data management compared to document-based e-pedigree systems.
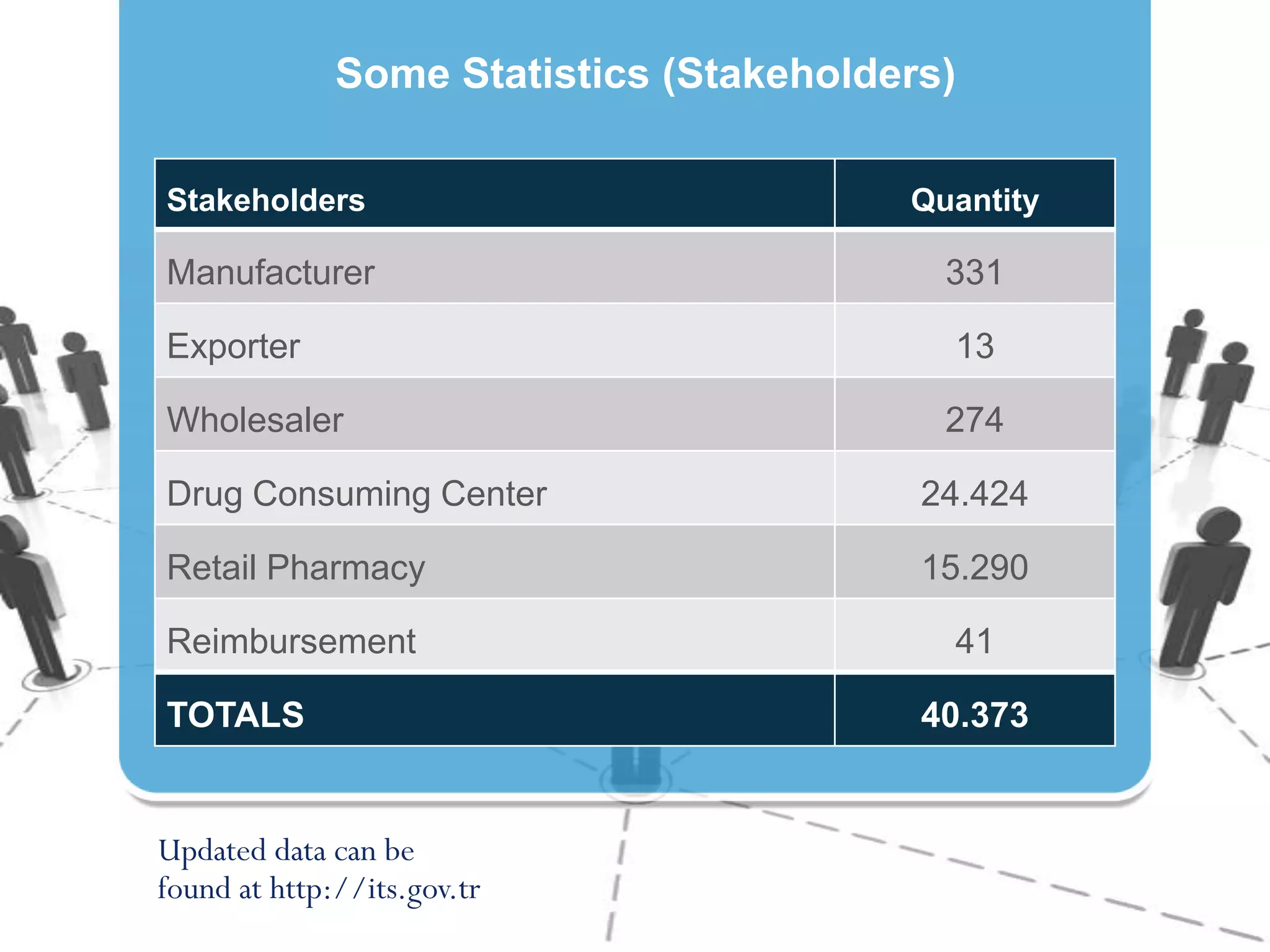
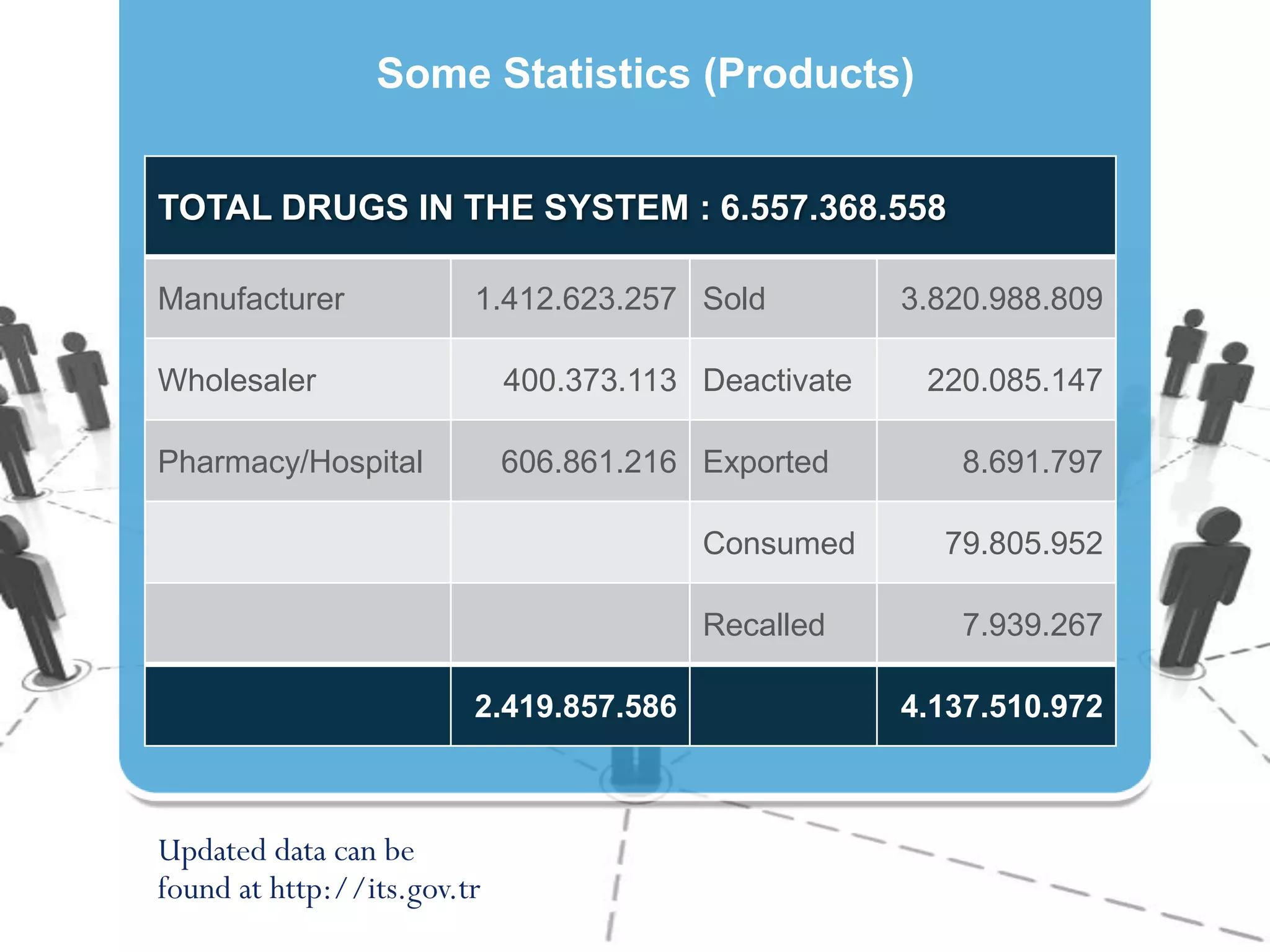
4) Producing statistics on over 40,000 stakeholders and over 6.5 billion drug units tracked to date, demonstrating the system's effectiveness.