The document summarizes key concepts about the nature of atoms and molecules, including:
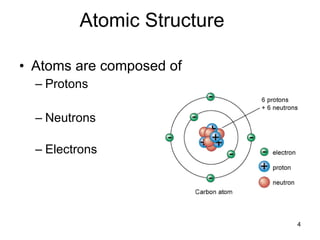
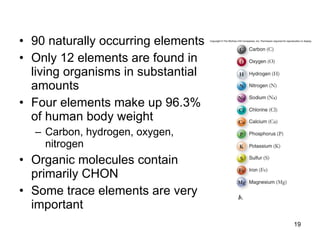
- Atoms are composed of protons, neutrons, and electrons. The number of protons determines the element.
- Electrons can be gained or lost, creating ions with positive or negative charges.
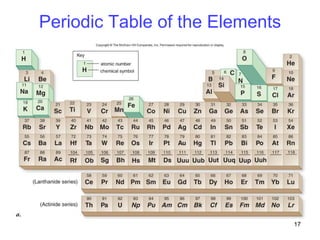
- Elements are arranged in the periodic table based on their atomic structure.
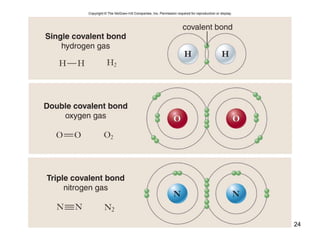
- Chemical bonds, including ionic and covalent bonds, determine how atoms combine to form molecules.