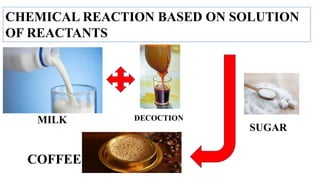
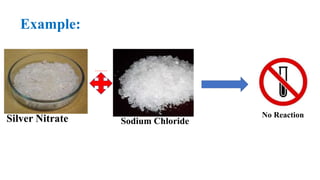
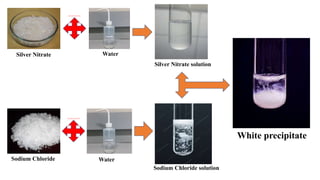
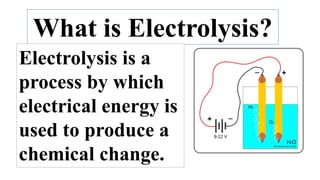
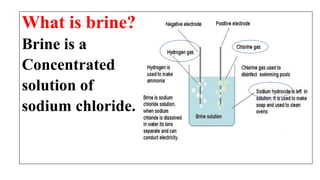
This document discusses chemical reactions that occur in solution form versus solid form and reactions that occur through the application of electricity. It provides the example of a chemical reaction between silver nitrate and sodium chloride solutions that produces a white precipitate, showing that some reactions only occur in solution. It then discusses how electricity can be used to cause chemical reactions, like the electrolysis of water into hydrogen and oxygen gases when an electric current is passed through water with sulfuric acid.