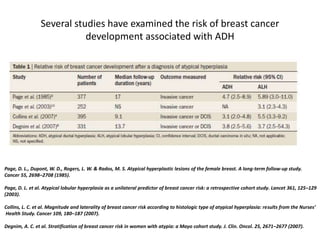
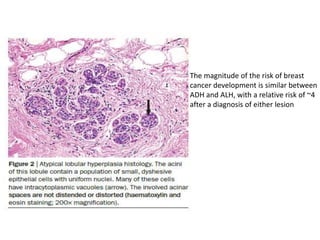
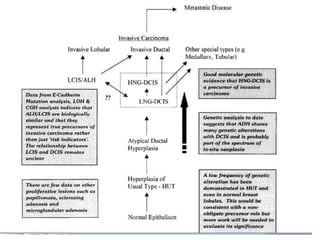
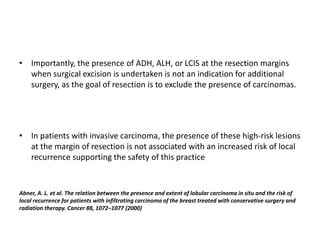
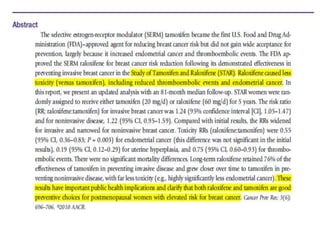
Proliferative breast disorders with atypia discusses various high-risk breast lesions including atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), lobular carcinoma in situ (LCIS), and ductal carcinoma in situ (DCIS). These lesions are associated with an increased risk of developing invasive breast cancer. The document outlines management strategies including active surveillance with screening, chemoprevention, and prophylactic mastectomy. While surgical excision is usually recommended for ADH, the management of ALH and LCIS remains debated due to their lower risk levels and potential for overtreatment.