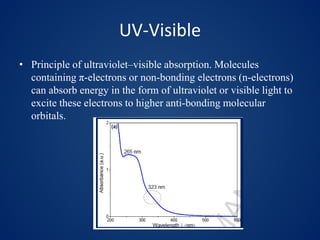
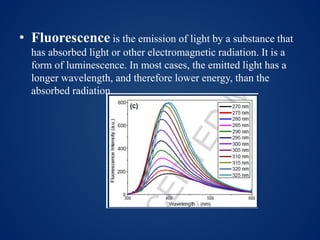
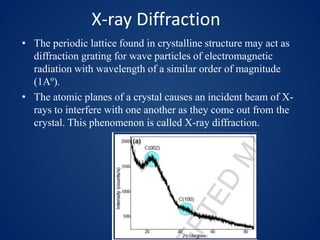
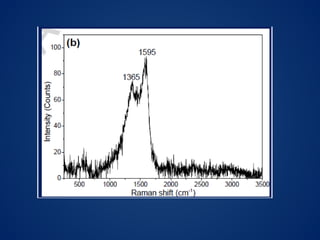
This document discusses carbon dots (CDs), which are small carbon nanoparticles less than 10nm in size with surface passivation. CDs were discovered in 2004 and have since been studied for their fluorescent properties. They are biocompatible and photostable. The document discusses methods for synthesizing CDs and characterizing their properties with techniques like UV-Vis, fluorescence, TEM, and Raman spectroscopy. It also discusses applications of CDs in areas like bioimaging, optronics, catalysis, drug delivery, and biosensing. Specifically, it notes CDs are being studied for optical imaging applications and targeting cancer cells.


















![• Two-photon excited fluorescence images of MCF-
7 cancer cells after incubation with FTNP0 (A) and
FTNP40 (B) for 2 h at 37 1C ([T1] = 1 mM). The
images were recorded upon 800 nm excitation
with a 505 nm longpass barrier filter.](https://image.slidesharecdn.com/cd-190121075254/85/Carbon-dots-characterization-and-applications-24-320.jpg)
