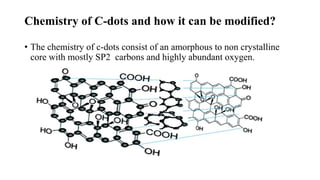
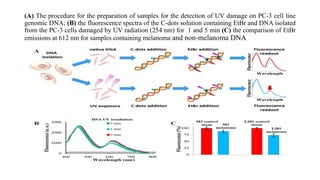
Carbon dots (C-dots) were used to detect UV-induced DNA damage. C-dots are small carbon nanoparticles less than 10nm in size with surface passivation that gives them fluorescent properties. In this study, C-dots were modified with amine groups to give them a positive charge and ability to interact with negatively charged DNA. Genomic DNA was isolated from human cells and exposed to UV radiation for varying times. C-dots were incorporated into both native and UV-exposed DNA. Fluorescence energy transfer between the C-dots and ethidium bromide (EtBr) showed that the intensity of EtBr emission decreased more for DNA exposed to UV, indicating the C-dots method could more sensitively detect