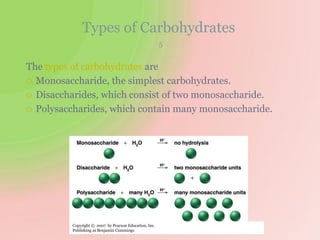
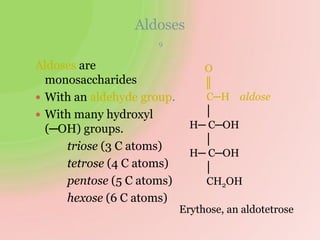
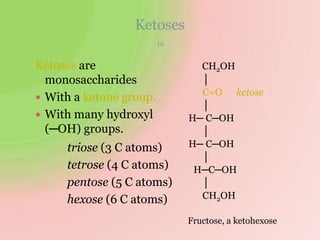
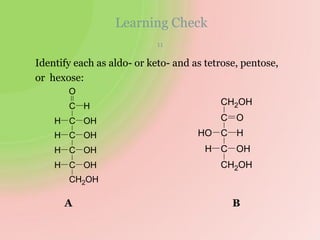
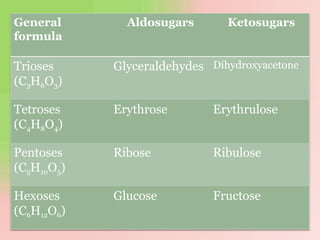
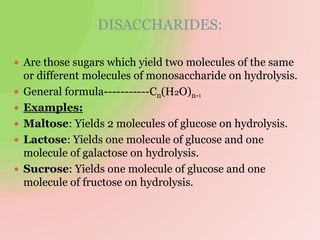
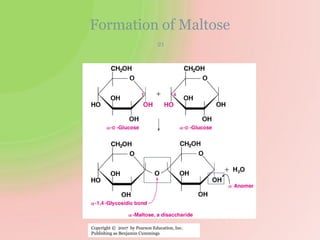
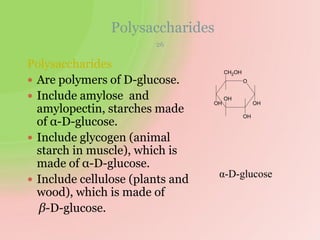
The document discusses carbohydrates, which are a major source of energy composed of carbon, hydrogen, and oxygen. They include monosaccharides (simple sugars), disaccharides (two monosaccharides joined), oligosaccharides (3-10 monosaccharides), and polysaccharides (long chains of monosaccharides). Important monosaccharides include glucose, fructose, and galactose. Disaccharides formed from two monosaccharides include maltose, lactose, and sucrose. Polysaccharides are polymers of monosaccharides and include starch, glycogen, and cellulose. Carbohydrates have important biomedical roles as energy sources and structural components in the body.