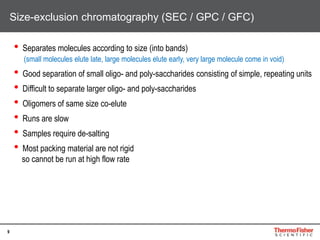
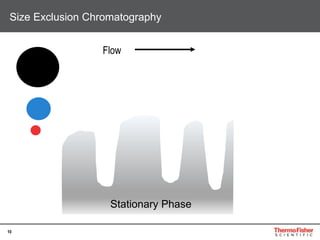
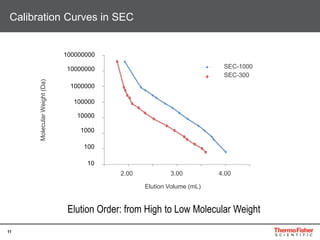
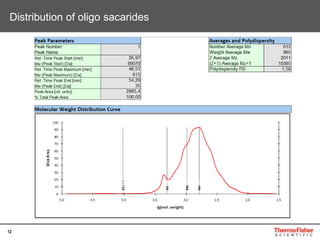
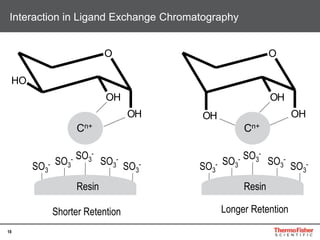
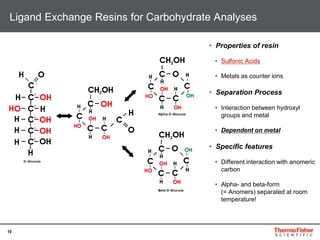
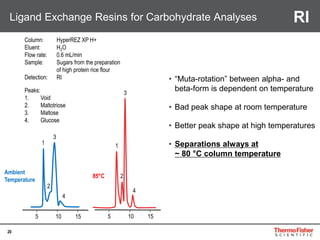
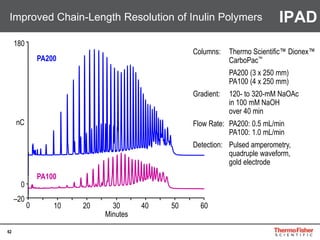
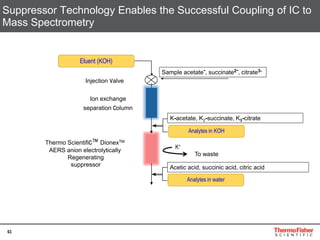
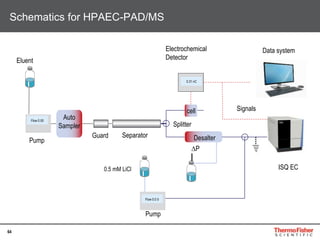
This document provides an overview of various chromatography techniques for analyzing carbohydrates, including their principles and applications. It discusses size exclusion chromatography, ligand exchange chromatography, reversed-phase chromatography, hydrophilic interaction liquid chromatography (HILIC), and high-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD). The presentation focuses on selecting the appropriate technique based on the properties of the carbohydrates being analyzed and highlights considerations for optimizing the separation and detection of carbohydrates.



![65
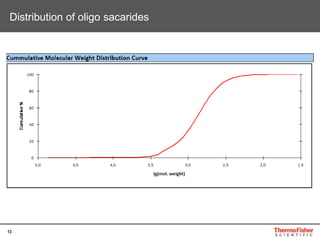
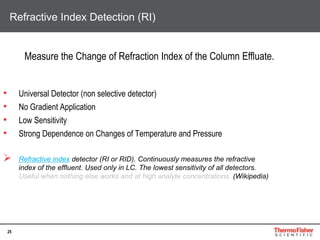
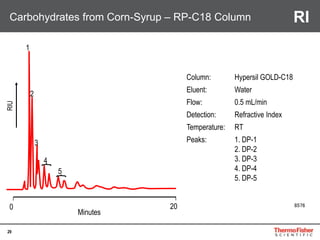
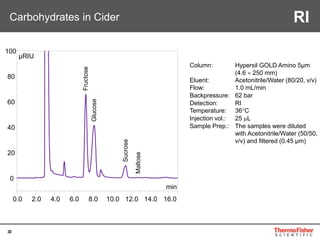
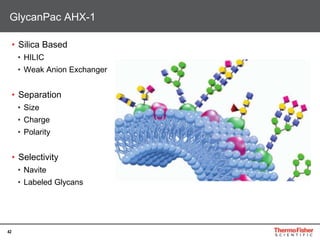
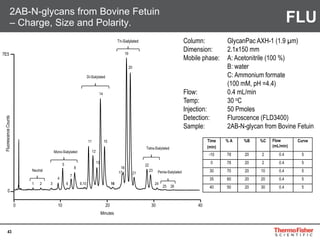
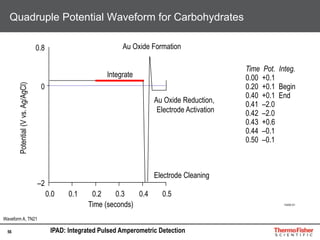
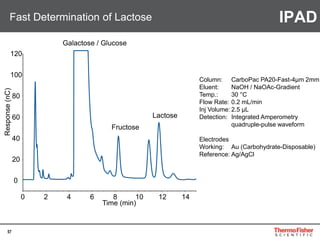
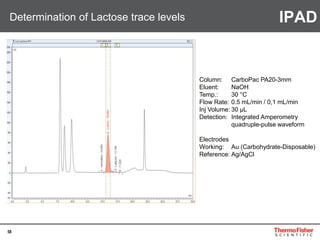
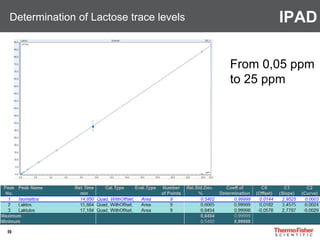
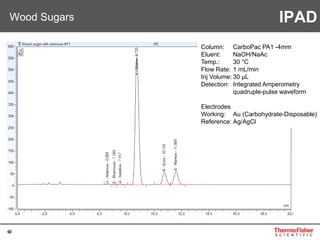
Detection of DP3 in Inuline
65
–10
100
250
nC
0 10 20 30 40 50
–25 000
200 000
400 000
counts
Minutes
510.50–511.50 m/z
GF2 F3
673 GF3
835 GF4
0
50
120
150 200 250 300 350 400 450 500 550 600
DP3a AV: 8.18-8.53 min (11) SB: 5.91-6.74 (25) NL: 2.64E5 T: {0,0} + c ESI corona sid=100.00 det=1129.00 Full ms ...
511
331
349
169
205 253
%
[Hex3+Li]+
[Hex2 + Li]+
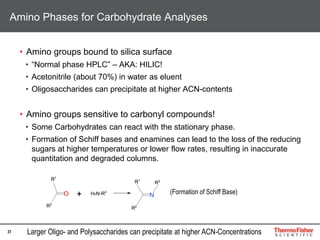
[Hex2 – H2O + Li]+
[Hex1 – H2O + Li]+
187
[Hex1 + Li]+
[Hex1 + H2O Li]+
Extracted Ion Chromatogram
IPAD Trace
20907a
MS](https://image.slidesharecdn.com/carbohydratesolutions40min-190214163104/85/Carbohydrate-solutions-40-min-62-320.jpg)