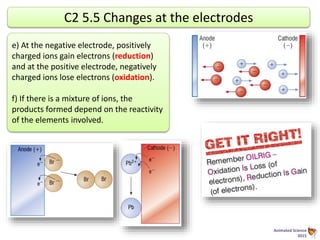
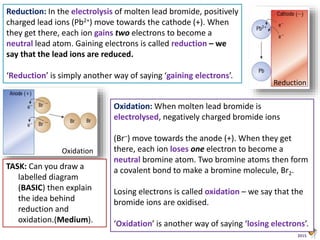
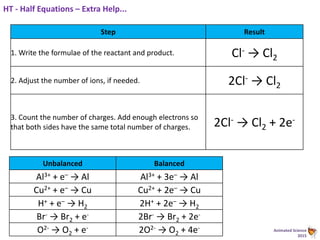
During electrolysis, ions move towards the electrodes. Positively charged ions move towards the negative cathode, where they gain electrons through reduction. Negatively charged ions move towards the positive anode, where they lose electrons through oxidation. In the electrolysis of molten lead bromide, lead ions are reduced at the cathode by gaining electrons, while bromide ions are oxidized at the anode by losing electrons to form bromine gas.