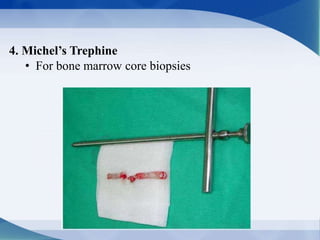
The document discusses bone marrow biopsy procedures. It describes:
- Bone marrow as the flexible tissue within bone cavities that can be red (involved in blood cell production) or yellow (non-productive).
- Indications for bone marrow biopsy including abnormal blood counts, infections, cancers, and iron storage investigations.
- Precautions for biopsy in cases of bleeding disorders, anemia, or low platelet counts.
- The steps of bone marrow biopsy including equipment, patient preparation, sample collection and evaluation, and sample submission.