Embed presentation
Download to read offline
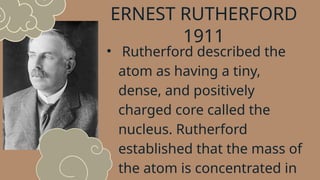
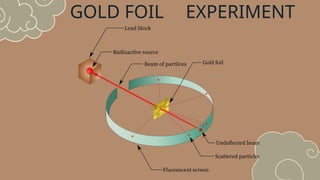

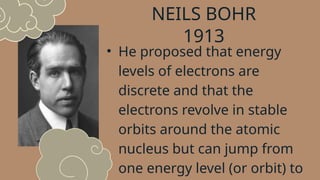

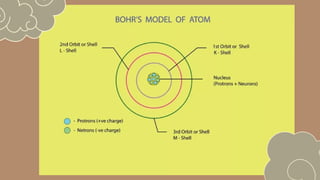











The document discusses various atomic models, including Rutherford's discovery of the nucleus and Bohr's proposal of discrete energy levels for electrons. It highlights the differences between Rutherford's and Bohr's models, specifically regarding electron orbits, and describes Schrödinger's electron cloud model as the most advanced representation of atomic structure. The importance of these atomic theories in shaping modern science, including chemistry and nuclear energy, is also emphasized.
















