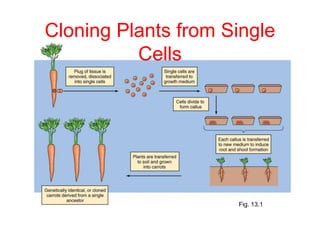
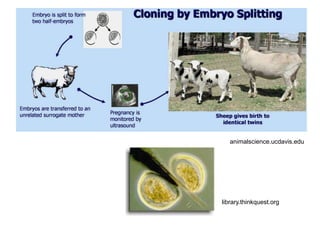
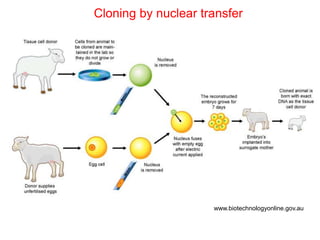
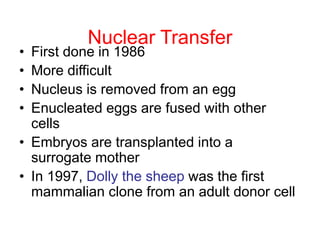
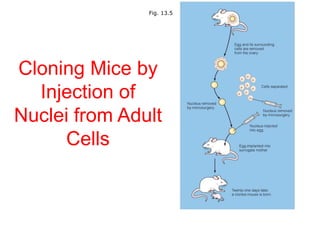
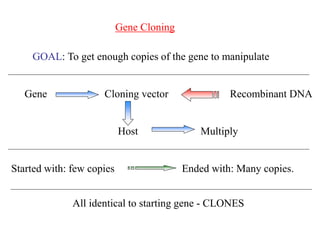
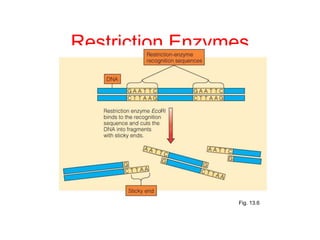
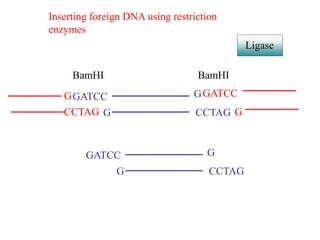
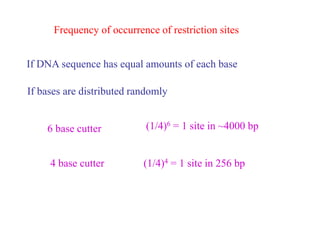
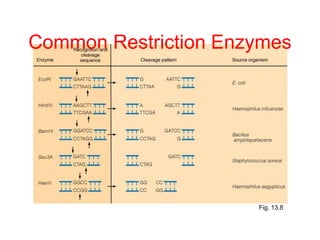
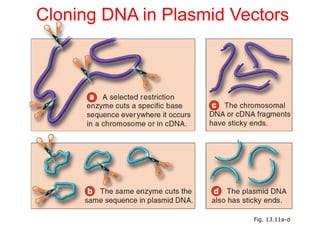
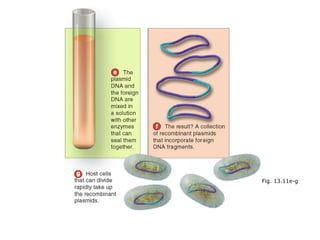
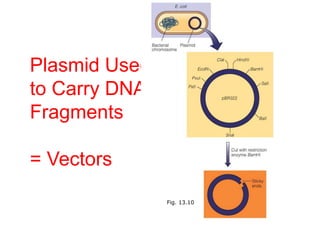
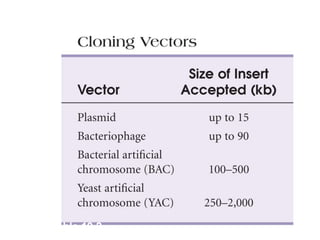
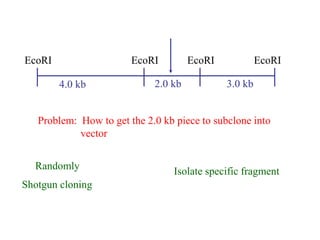
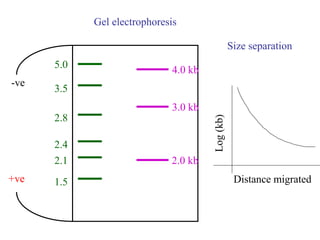
This document discusses cloning techniques including cloning plants from single cells and cloning animals using embryo splitting and nuclear transfer. Embryo splitting involves fertilizing an egg in vitro, growing it to 8-16 cells, separating the cells, and transplanting them into surrogate mothers. Nuclear transfer removes the nucleus from an egg, fuses it with another cell, and transplants the embryo into a surrogate. Dolly the sheep was the first mammal cloned using nuclear transfer. Gene cloning involves using restriction enzymes and vectors like plasmids to multiply copies of a gene for manipulation. Steps in cloning a single piece of DNA include isolating the DNA fragment, ligating it into a cut vector, transforming bacteria, and confirming the recombinant plasmid.