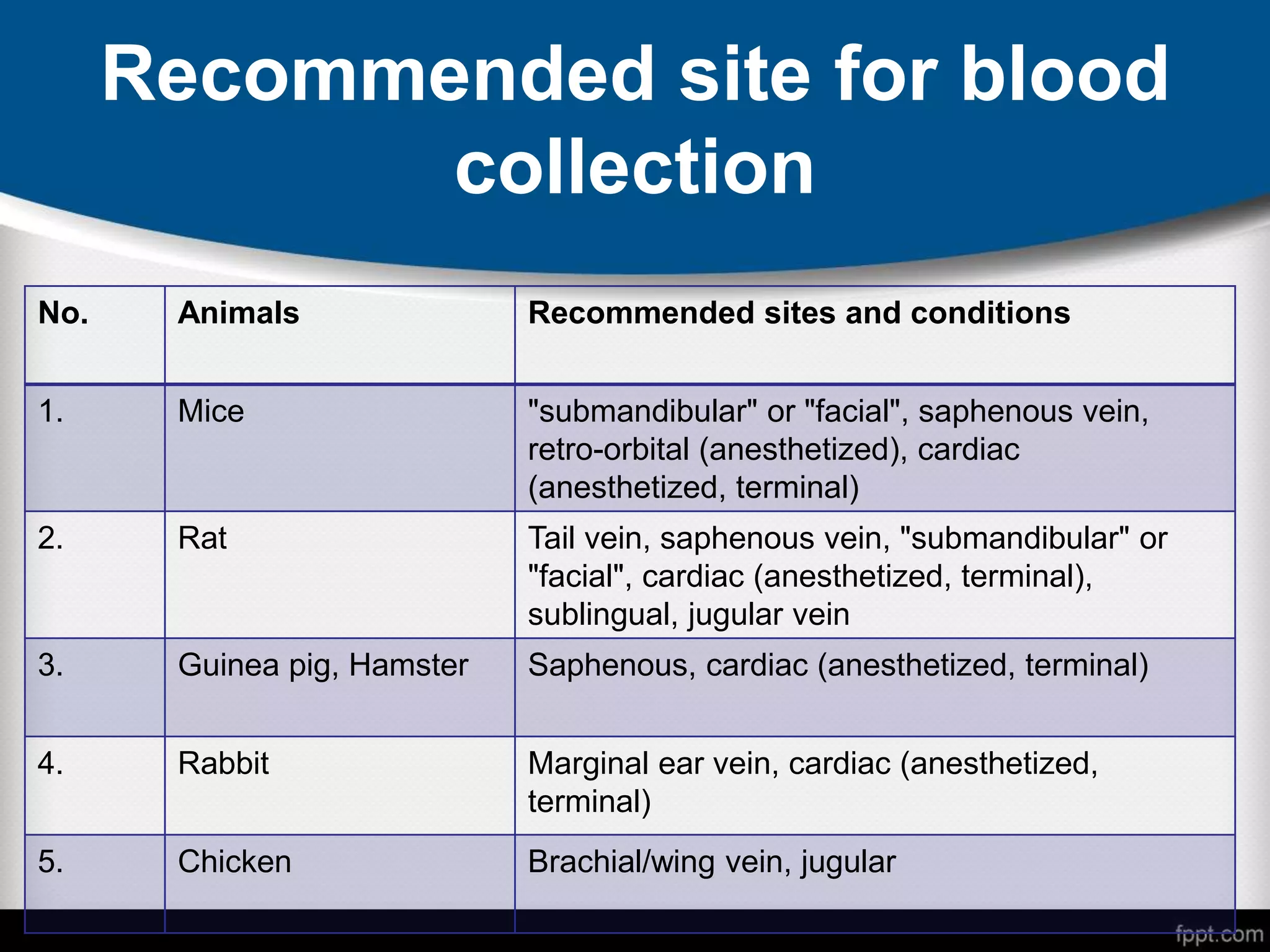
This document provides guidelines for blood sample collection in small laboratory animals, emphasizing the necessity of ethics approval and minimizing pain and stress during the procedure. It details methods of blood collection, common anesthetic agents, and specific techniques for various species while also specifying recommended sites, volumes, and frequencies for blood withdrawal. The document concludes with references for further reading on blood collection practices in laboratory settings.






![References
• Hoff J, Rlagt LV. Methods of blood collection in the mouse. Lab
animals 2000;29:47-53.
• Blood sampling online. 2009; [12 screens]. Available
from:http://www.nc3rs.org.uk/bloodsamplingmicrosite/page.asp?id=3
13[Last cited on 2010 Fe24].
• Guidelines for Survival Bleeding of Mice and Rats. NIH-ARAC
Guidelines [online]. 2005 Jan 12. Available from
http://oacu.od.nih.gov/ARAC/Bleeding.pdf [accessed on 2010 Mar
09].
• McGuill MW, Rowan AN. Biological Effects of Blood Loss:
Implications for Sampling Volumes and Techniques. ILAR J 1989. p.
31](https://image.slidesharecdn.com/bloodsamplecollectionmethod-230707063314-12001a4e/75/Blood-Sample-Collection-Method-ppt-28-2048.jpg)
![Continue…
• Procedure for rabbit blood collection [online]. Available from:http://
www.research.uky.edu/ori/univet/resources/sop/Procedure_rabbit_bl
ood_ collection.pdf [Last cited on 2010 Feb 23].
• CPCSEA guidelines for laboratory animal facility. Indian J
Pharmacol 2003;35:257-74.
• Vogel HG, editor. Drug discovery and evaluation: Pharmacological
assays. 2nd ed. Berlin: Springer; 2002.
• Anaesthesia and Analgesia in Laboratory Animals at UCSF [online].
Available from :http://www.iacuc.ucsf.edu/Index.asp[Last cited on
2010 Feb 24]. and Availabl
from:http://www.iacuc.ucsf.edu/Proc/awRatFrm.asp. [Last cited on
2010 Feb 24].](https://image.slidesharecdn.com/bloodsamplecollectionmethod-230707063314-12001a4e/75/Blood-Sample-Collection-Method-ppt-29-2048.jpg)
