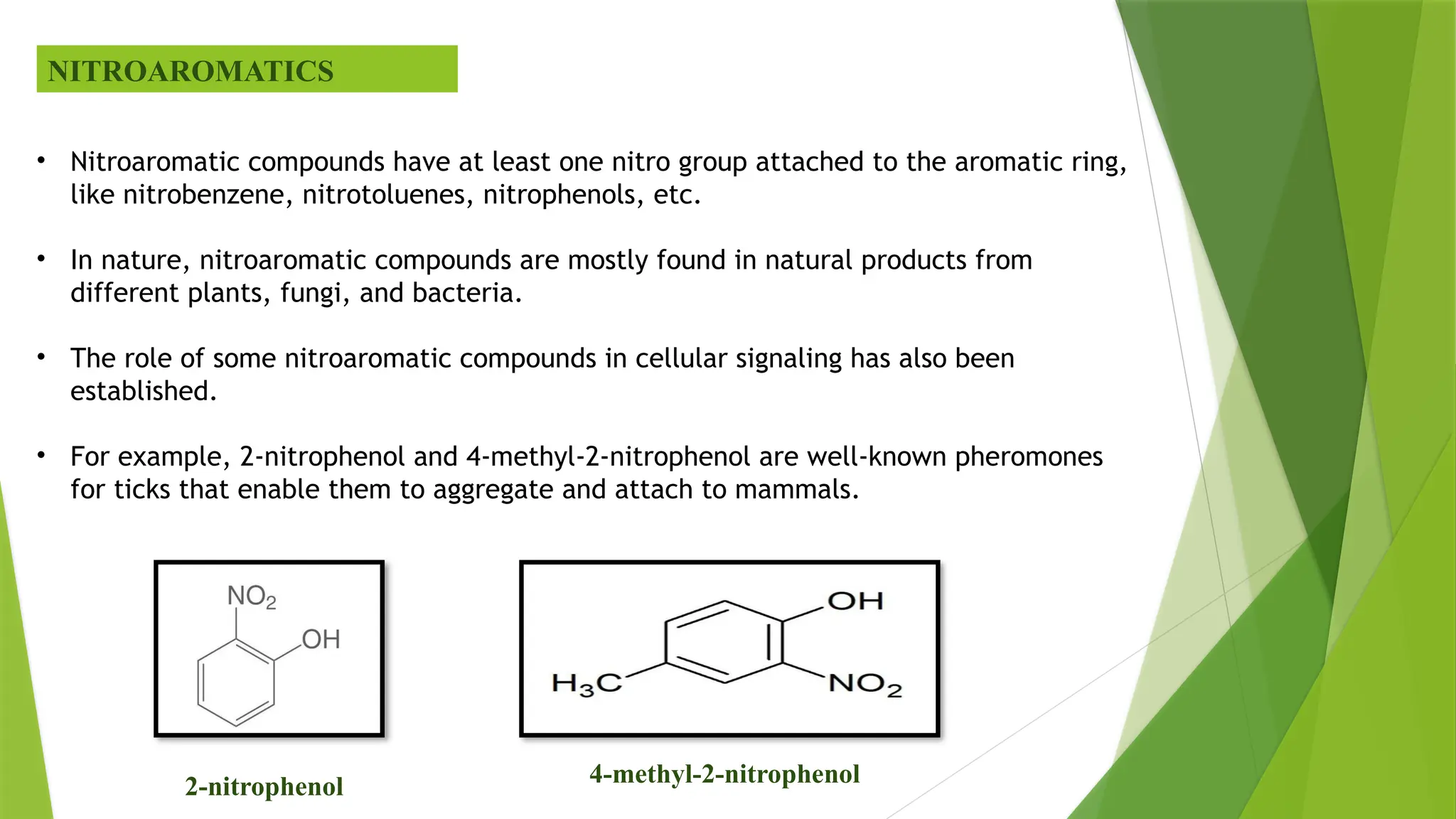
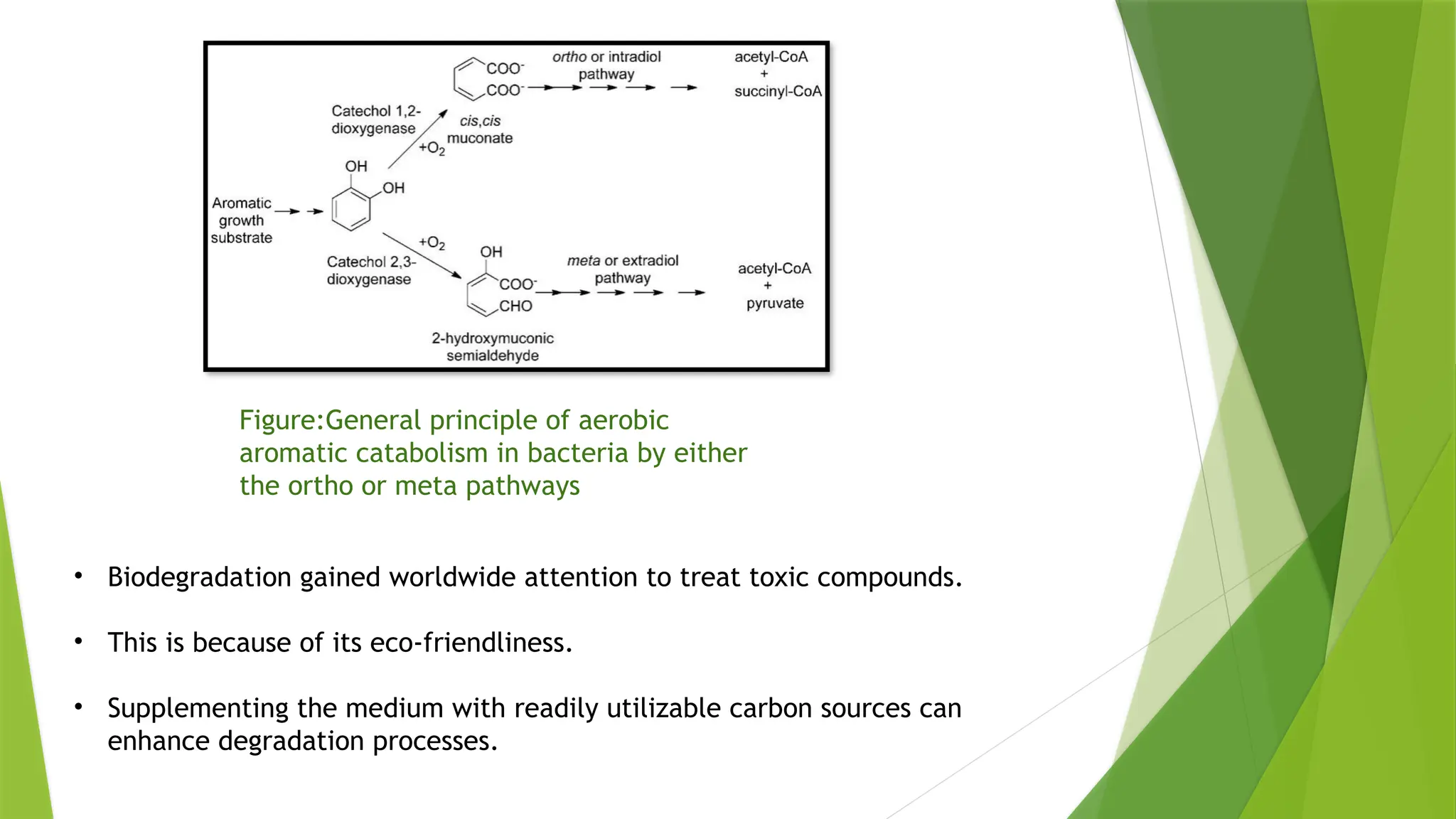
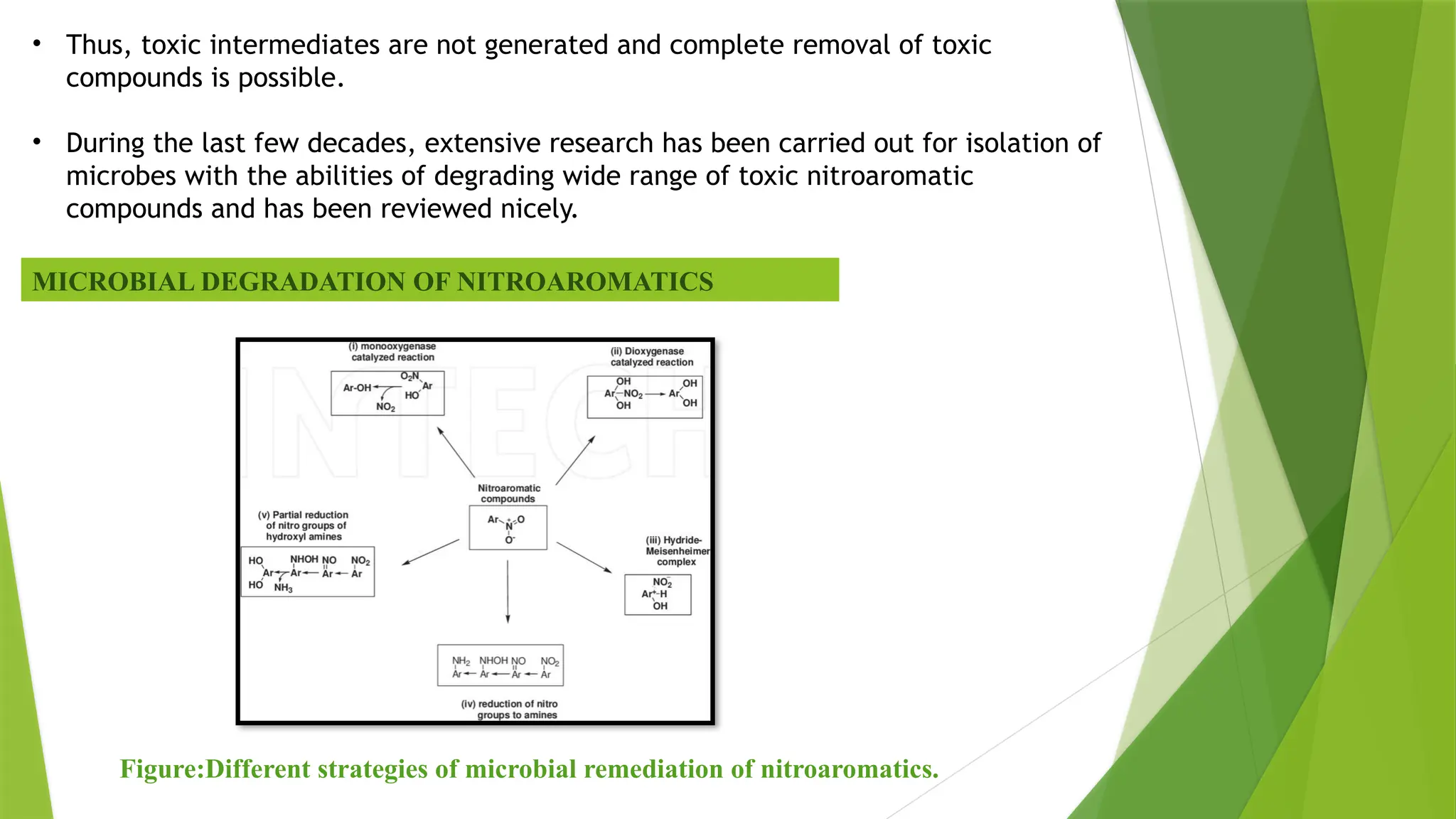
The document discusses the degradation of nitroaromatic compounds and their impact on the environment, highlighting their synthetics, toxicity, and methods for bioremediation. It outlines the role of microbes in degrading these pollutants through various metabolic processes, emphasizing aerobic and anaerobic biodegradation, as well as the evolution of bacterial genes for nitroaromatic degradation. The overall conclusion points to the urgent need for effective bioremediation strategies to address the environmental hazards posed by these compounds.