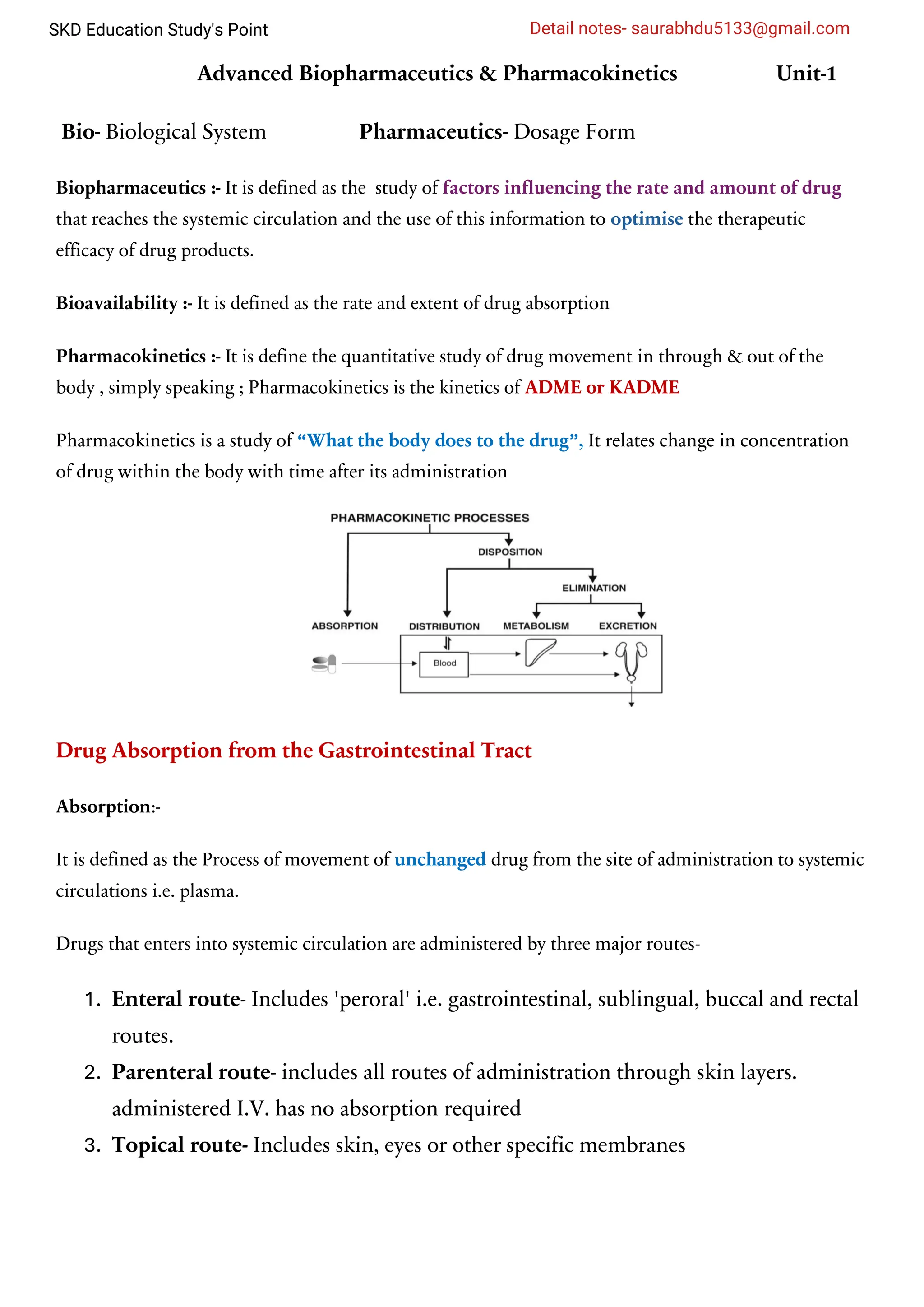
Hi everyone in this document I will try to cover biopharmaceutics in more intresting way I hope you like it if you want this notes than contact with me in our telegram group -SKD Pharma Education
This notes also helpful in different compitetive exam such as NIPER, GPAT, Gvt.Pharmacist, DI
This is for B.Pharm and M.Pharm both condidate I will follow PCI syllabus and we follow reference book as brahmankar

![Passive diffusion follows First order kinetics [GPAT-2015]
Passive diffusion is best expressed by Fick's first law of diffusion, which states that the drug molecules
diffuse from a region of higher concentration to one of lower concentration until equilibrium is
attained and that the rate of diffusion is directly proportional to the concentration gradient across
the membrane.
Mathematically Fick's law of diffusion is expressed by
where,
dQ/dt = rate of drug diffusion (amount/time).
D = diffusion coefficient of the drug through the membrane (area/time).
A = surface area of the absorbing membrane for drug diffusion (area).
Km/w = Partition coefficient of the drug between the lipoidal membrane and the aqueous GI Fluids
(no units)
(CGIT- C) = Difference in the concentration of drug in the GI fluids and plasma (amount/volume)
h = Thickness of membrane (length)
Due to sink conditions (CGIT >> C) the concentration of drug in plasma C is very small in comparison
to CGIT
Pore Transport
Also, called as convective transport, bulk flow or filtration
Driving force in this process is osmotic differences or hydrostatic pressure](https://image.slidesharecdn.com/biopharmunit-1-1-250311121814-9d08f6b2/75/Advanced-Biopharmaceutics-notes-for-M-Pharm-2nd-semester-Unit-1-pdf-M-Pharm-Pharmacy-3-2048.jpg)



