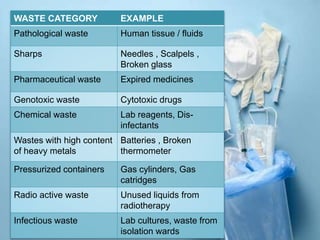
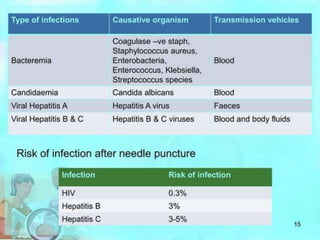
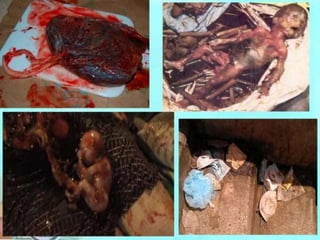
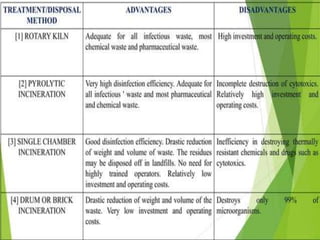
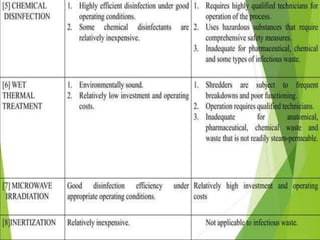
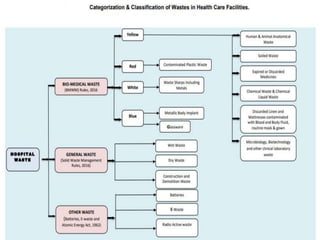
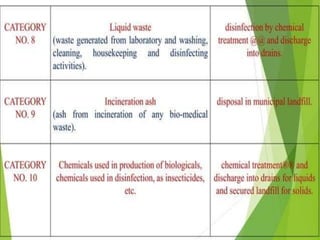
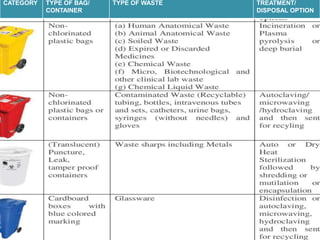
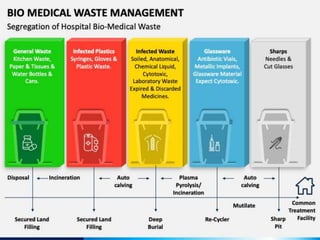
The document discusses healthcare waste and its management. It states that healthcare waste poses higher risks than other waste due to potential for infections. It also provides definitions of healthcare waste and categories such as infectious, sharp and pharmaceutical waste. The document outlines the major steps in healthcare waste management - segregation, collection, transportation, storage, treatment and disposal. It emphasizes the importance of proper waste handling and treatment to prevent health hazards.