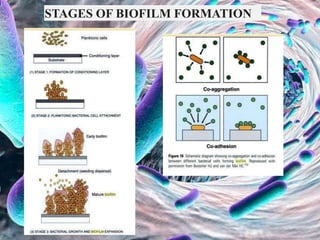
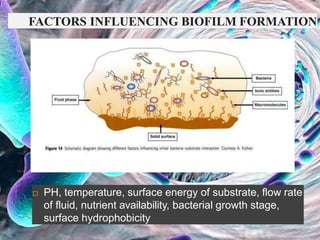
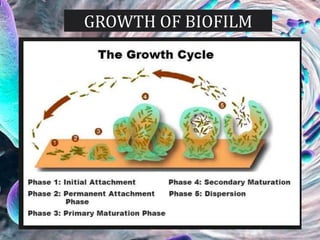
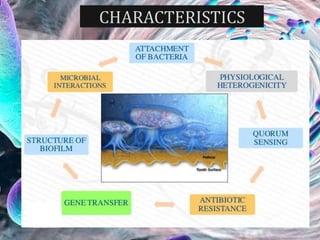
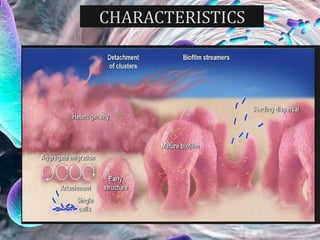
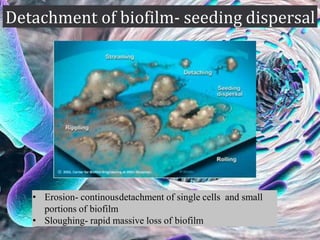
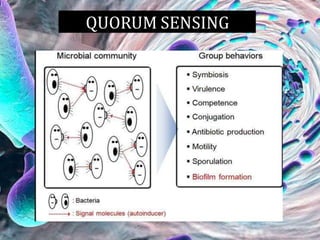
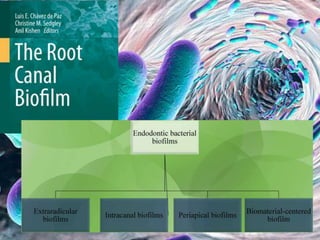
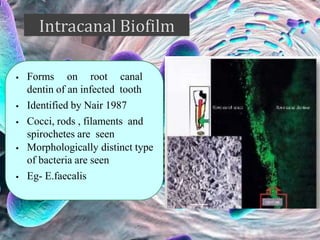
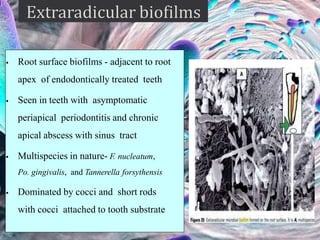
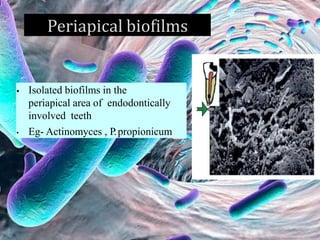
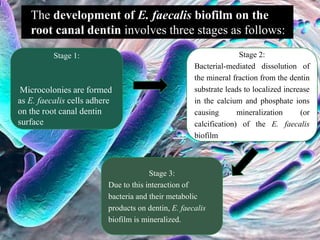
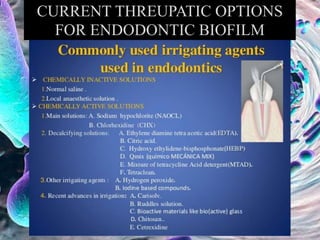
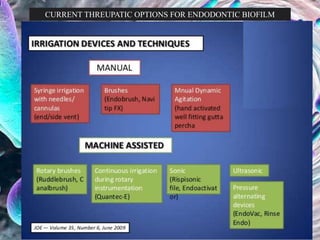
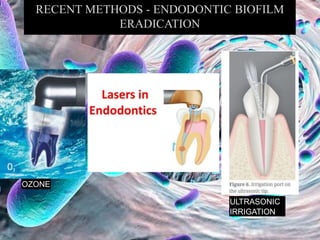
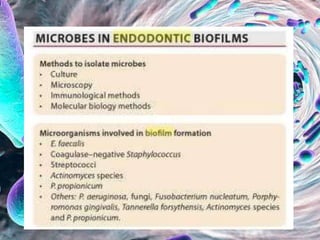
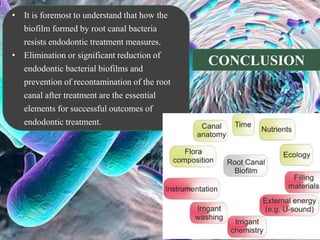
The document provides an in-depth analysis of biofilms in endodontics, detailing their structure, formation, and the specific role of Enterococcus faecalis in root canal infections. It discusses the ecological conditions in root canals that facilitate biofilm formation and outlines therapeutic approaches to manage these persistent infections. Key factors affecting biofilm resilience and the necessity for effective treatments are emphasized for successful endodontic outcomes.