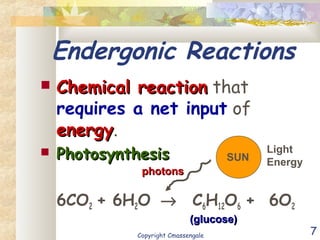
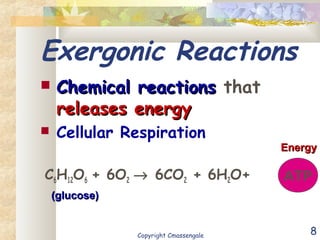
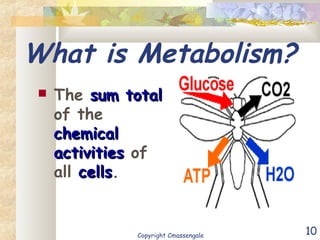
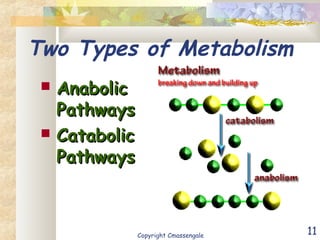
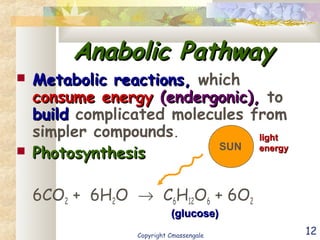
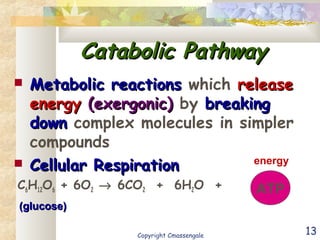
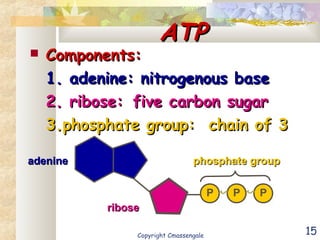
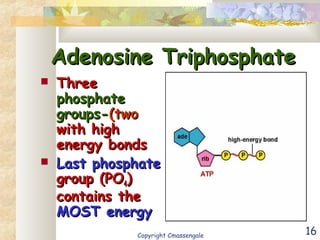
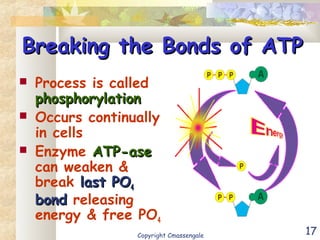
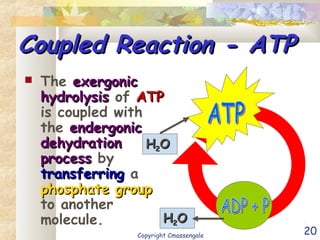
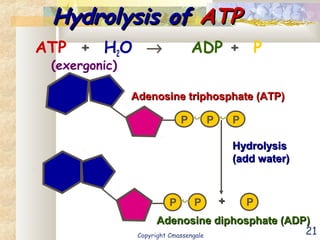
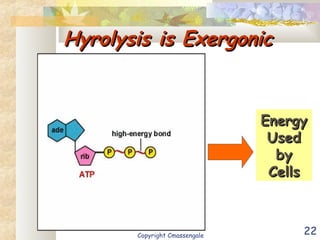
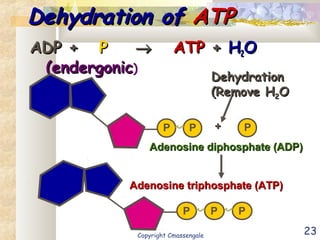
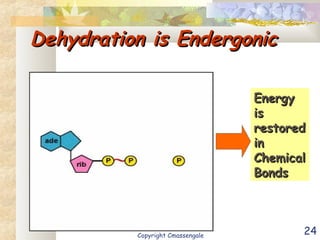
Bioenergetics is the study of energy in living systems and organisms. ATP (adenosine triphosphate) is the main energy carrier in cells. It contains high-energy phosphate bonds that are broken to release energy for cellular work through the hydrolysis of ATP. This exergonic reaction is coupled to endergonic cellular processes like biosynthesis through phosphorylation, transferring a phosphate group and restoring the chemical bonds in ATP.