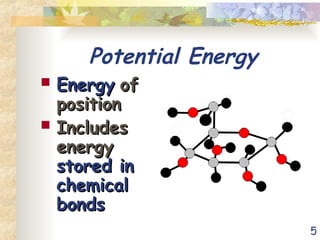
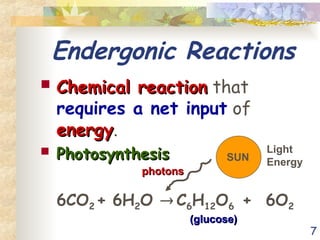
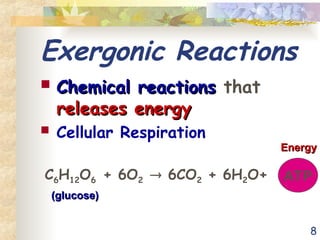
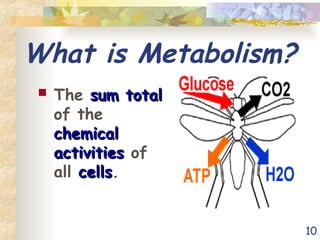
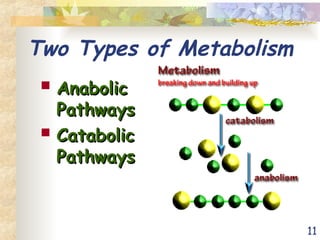
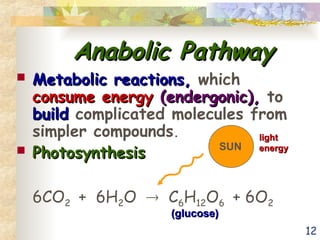
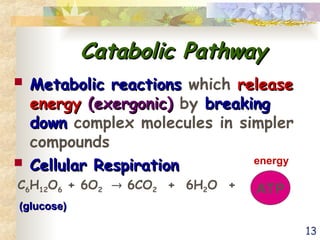
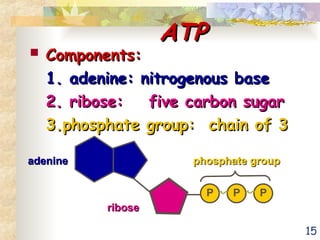
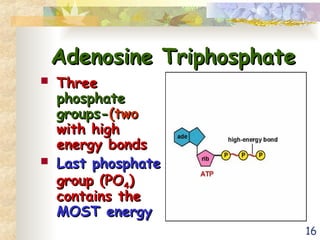
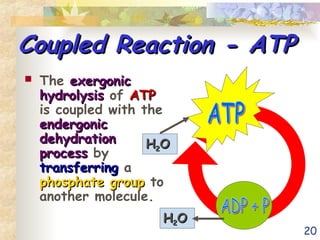
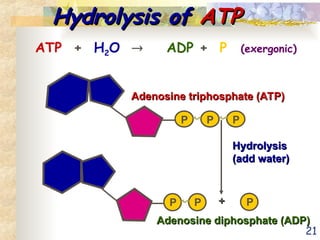
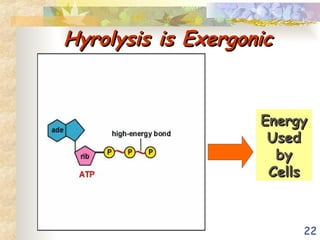
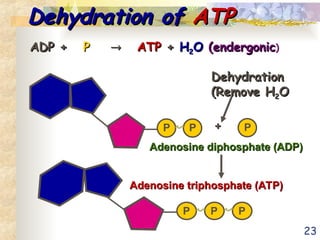
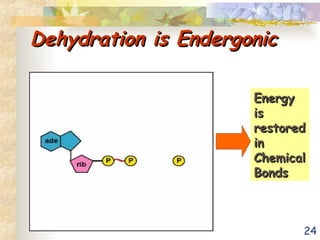
The document discusses bioenergetics, which is the study of energy in living systems and the metabolic processes of organisms. It outlines two types of energy reactions: endergonic (energy-consuming) and exergonic (energy-releasing), along with metabolism types: anabolic and catabolic pathways. Additionally, it explains the role of ATP as an energy currency in cells, detailing its structure, function, and the processes of hydrolysis and dehydration in energy transfer.