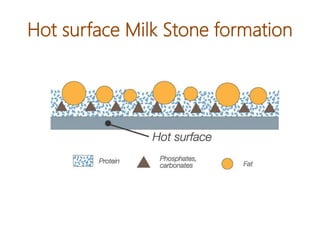
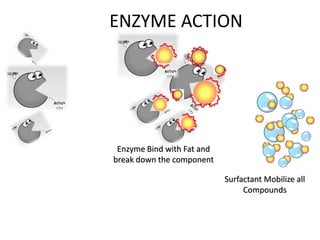
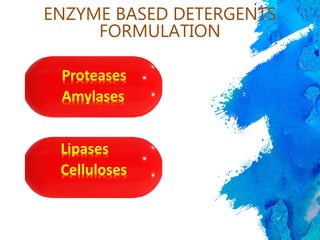
The document discusses the importance of cleaning agents in the dairy and food industry, highlighting the shortcomings of synthetic detergents, particularly in removing milk stones and their environmental impact. Enzyme-based detergents are emerging as a more effective and eco-friendly alternative, exhibiting greater efficiency and biodegradability. The document emphasizes the need for advanced cleaning solutions to address challenges like membrane fouling and biofilm formation in food processing environments.