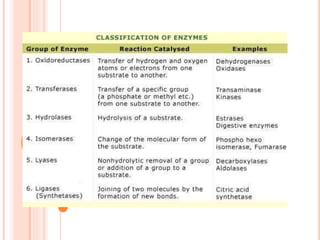
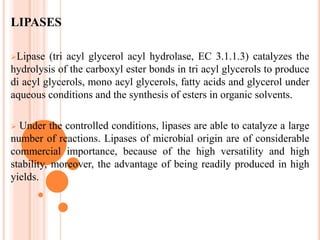
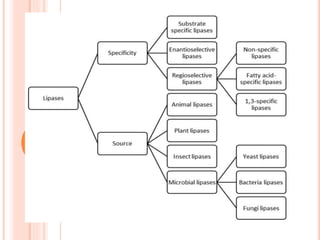
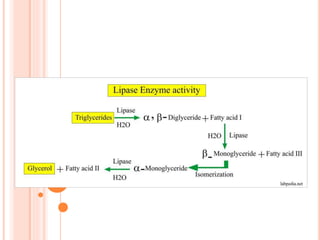
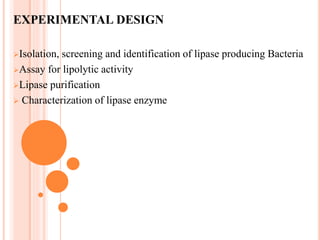
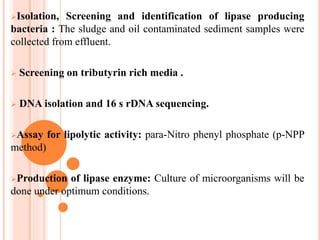
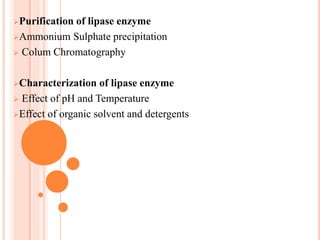
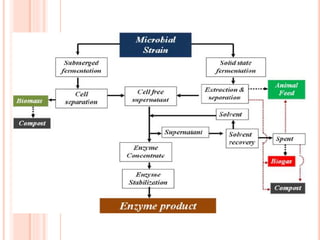
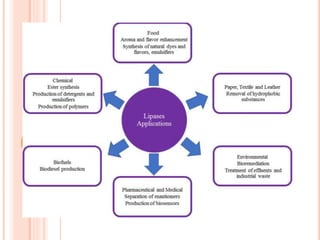
The document discusses lipase production and purification, including enzyme characteristics, classification, and industrial applications. It details the experimental design for isolating and screening lipase-producing microorganisms, as well as methods for enzyme production, purification, and characterization. Various applications of lipases in the food, pharmaceutical, and detergent industries are highlighted.