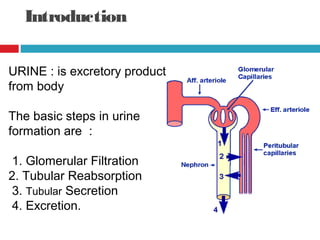
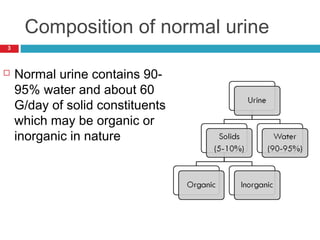
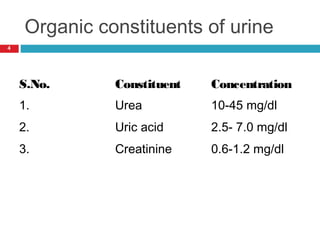
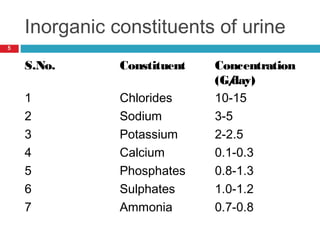
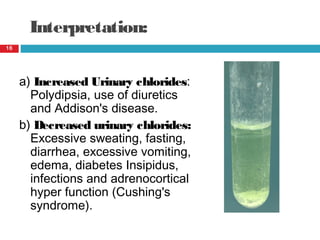
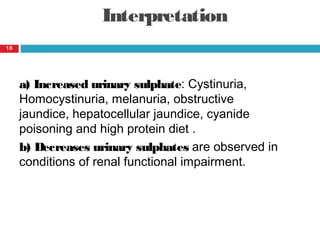
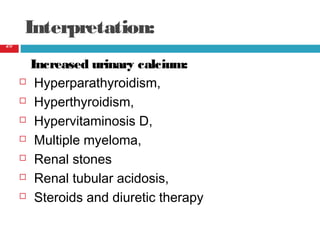
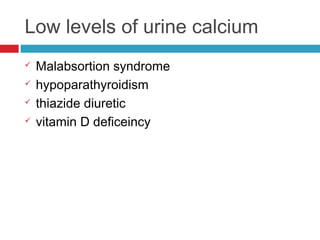
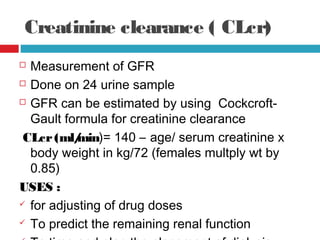
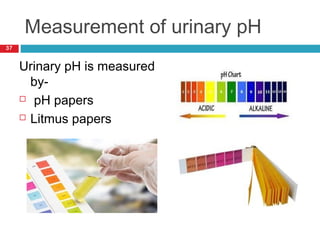
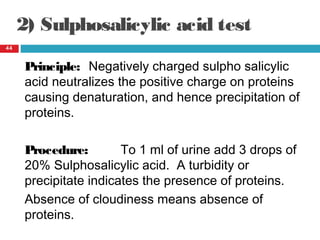
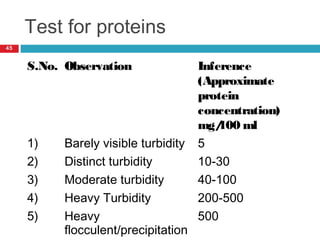
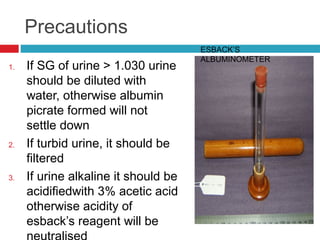
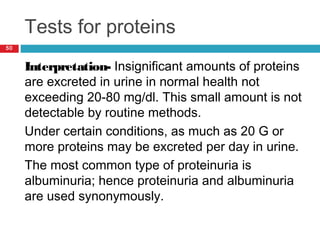
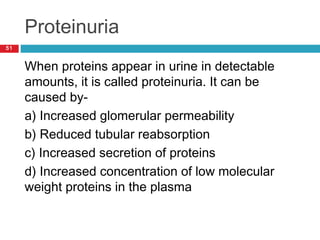
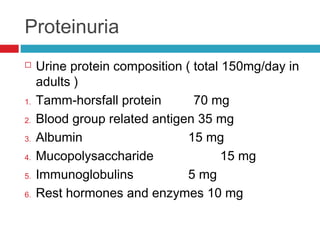
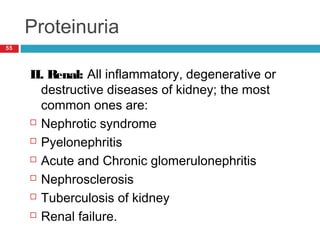
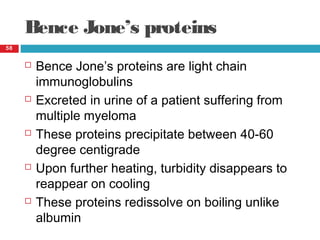
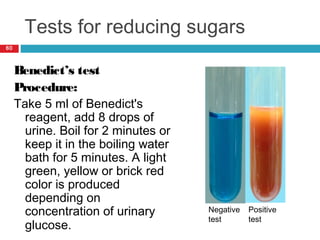
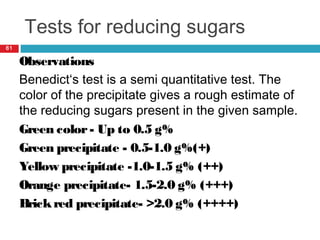
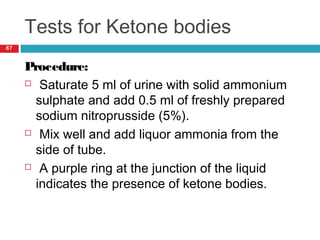
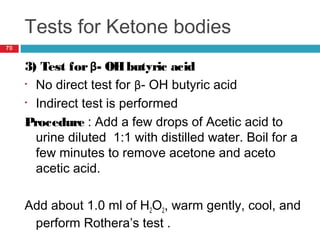
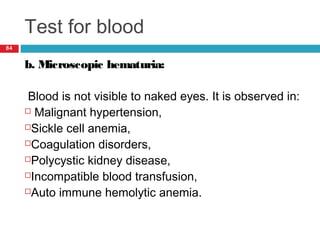
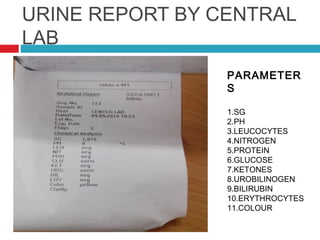
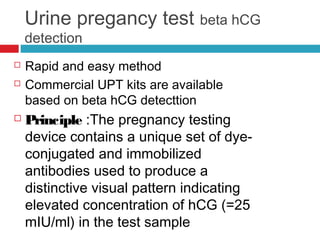
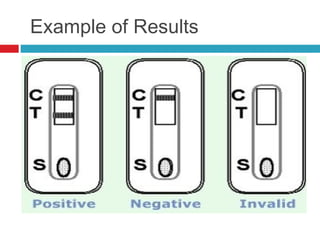
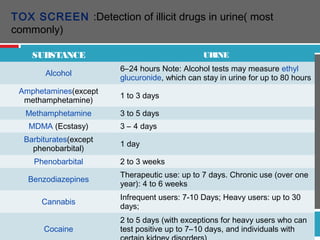
Urine biochemical analysis and its clinical significance was presented by Dr. Neeraj Nirala at RNT Medical College & MB Government Hospital. Urine analysis provides important information about kidney and systemic health by examining the urine's chemical composition, pH, and presence of abnormal constituents. The document discusses the normal organic and inorganic constituents of urine, various urine collection and storage methods, and qualitative and quantitative tests for analyzing urine components like pH, protein, glucose, ketones, bilirubin, urobilinogen, nitrites, blood, and drugs. Abnormal levels can indicate diseases of the kidneys, urinary tract, or other organ systems.