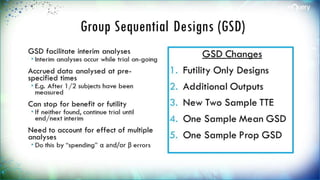
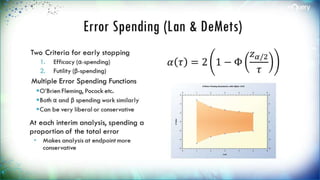
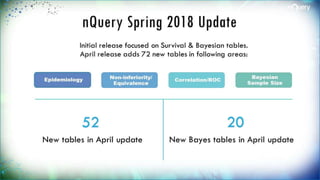
The document discusses adaptive trial designs, specifically focusing on sample size re-estimation (SSR) and its implications for clinical trials. It highlights the advantages and disadvantages of adaptive trials, the regulatory background supporting them, and the methodology behind blinded and unblinded SSR. The document also outlines recent updates to sample size calculation tools, emphasizing their widespread acceptance and expected growth in use.














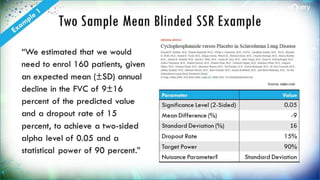
![Two Sample Proportion BSSR
Example
“For our sample size
calculations, we assumed that
20% of women in the control arm
would be using LARC after their
6-week postpartum visit, … An
analysis population with at least
626 women (313 in each arm)
was required to provide 80%
power (using a two-sided alpha
of 0.05) to detect an absolute
10% increase to 30% in LARC use
in the intervention arm [14].
Anticipating a maximum drop-
out rate of 20% at the time of
Follow-Up Survey #2, we planned
to randomize 800 participants”
Source: Contraception
Parameter Value
Significance Level (2-
Sided)
0.05
Control Rate 0.2
Intervention Rate 0.3
n per Group 313
Target Power (%) 80%
Nuisance Parameter Overall Success
Rate](https://image.slidesharecdn.com/innovativesamplesizemethodsforadaptiveclinicaltrialswebinar-webversion0-180926141725/85/Innovative-sample-size-methods-for-adaptive-clinical-trials-webinar-web-version-0-1-16-320.jpg)