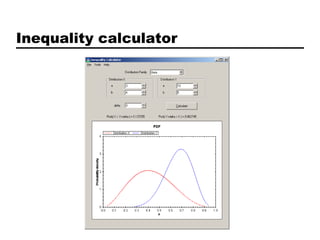
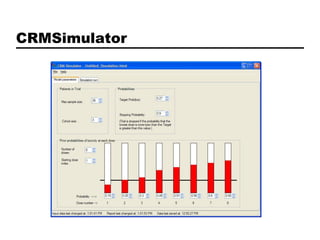
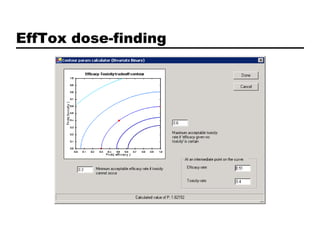
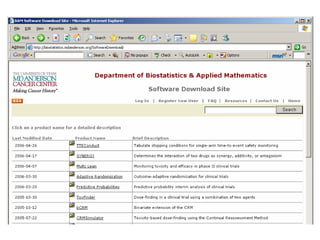
This document discusses Bayesian clinical trial software developed by John D. Cook and colleagues at M. D. Anderson Cancer Center. It describes software tools for designing trials, such as for sample size calculation and dose finding, and for conducting trials, such as for safety monitoring and adaptive randomization. Challenges in clinical trial conduct software design are also outlined, such as handling changes during a trial. Resources for downloading or learning more about the software are provided.