Embed presentation
Download as PDF, PPTX



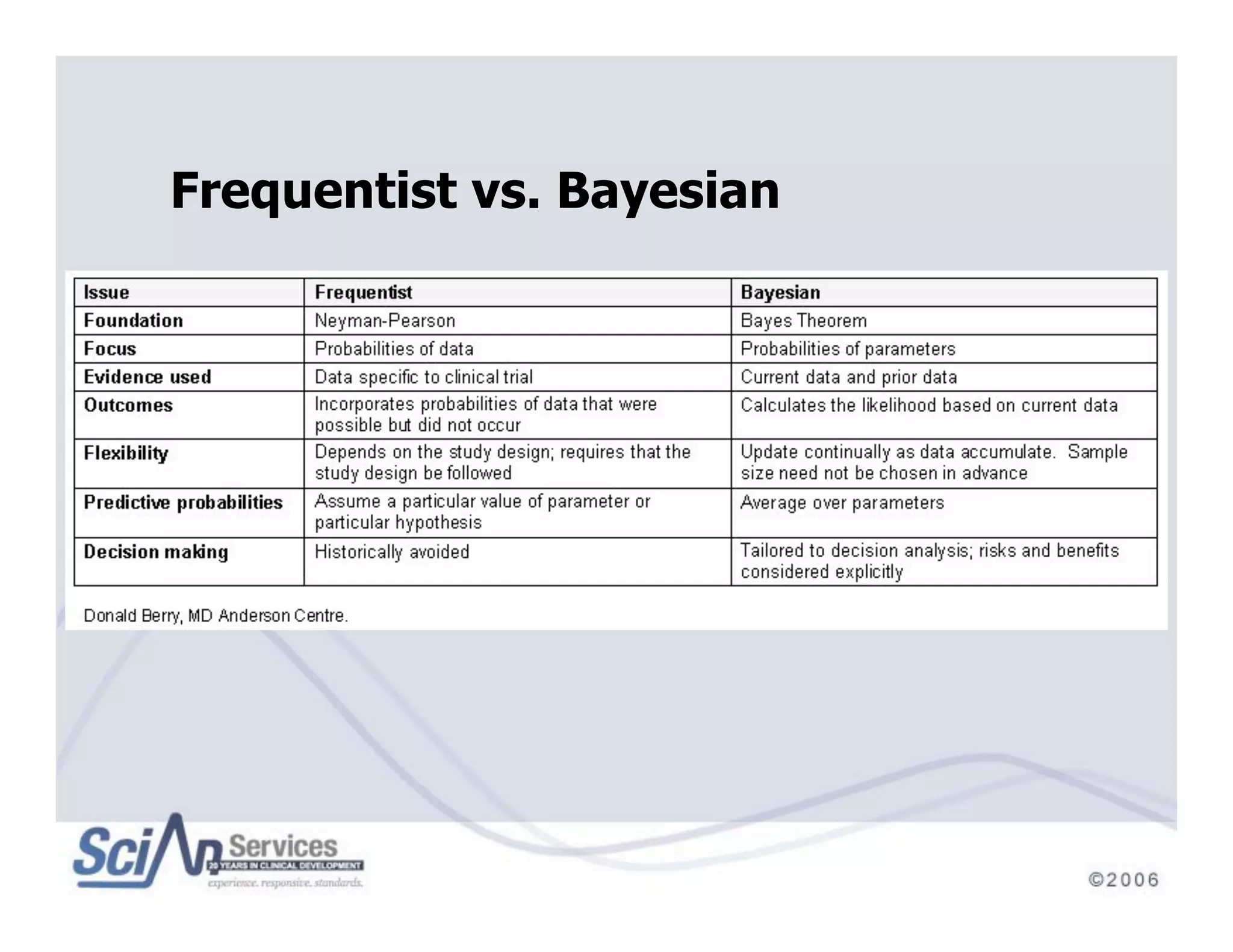
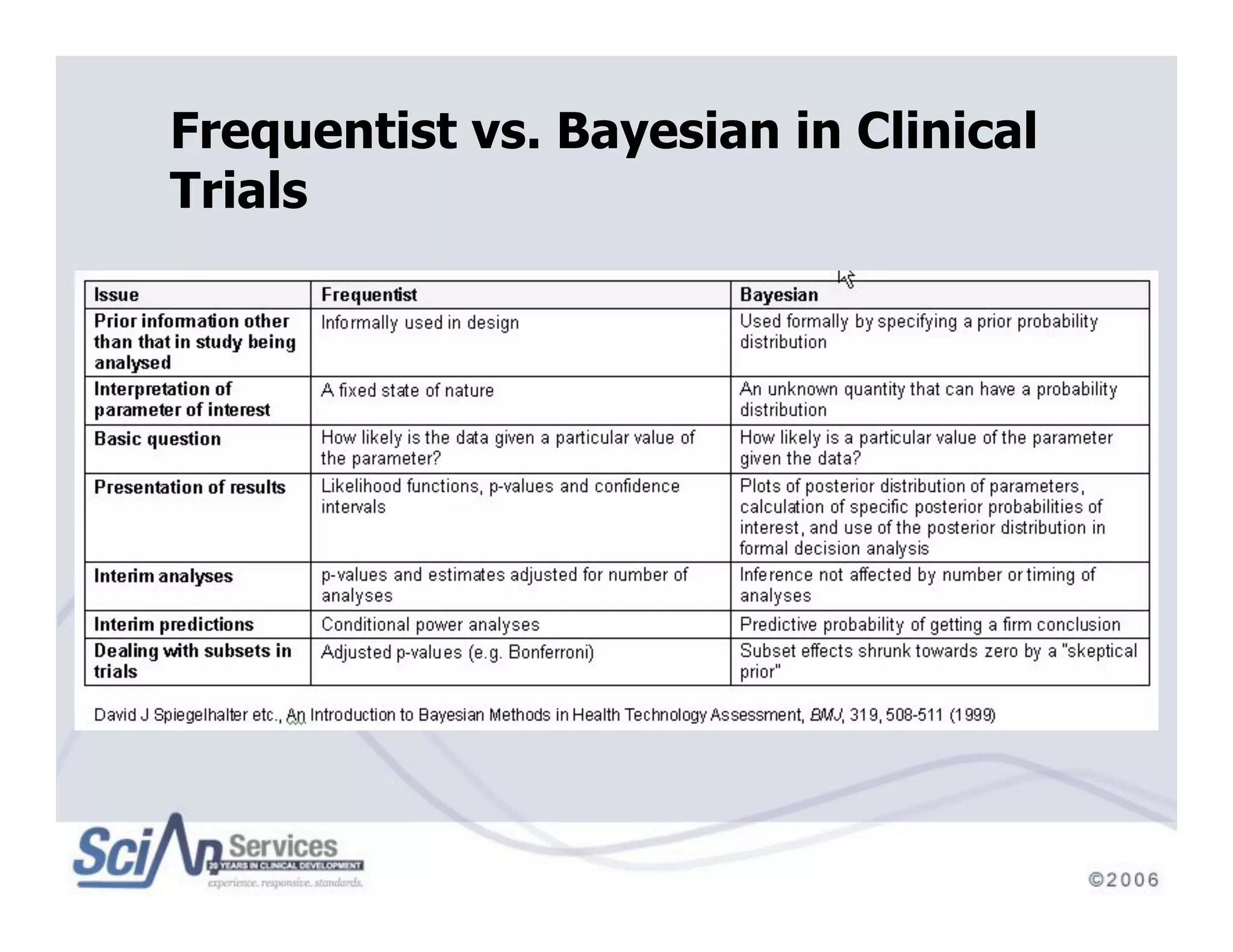
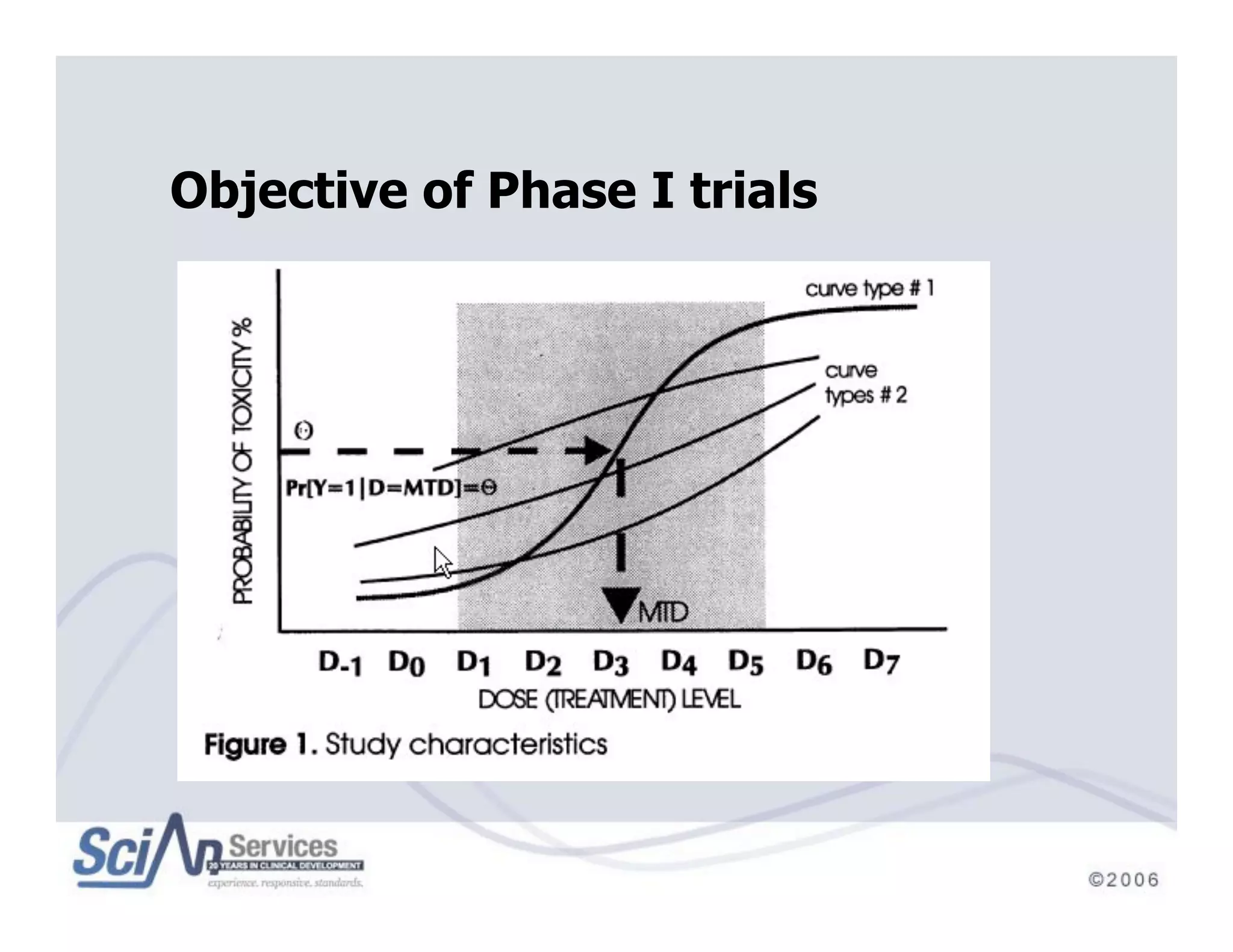
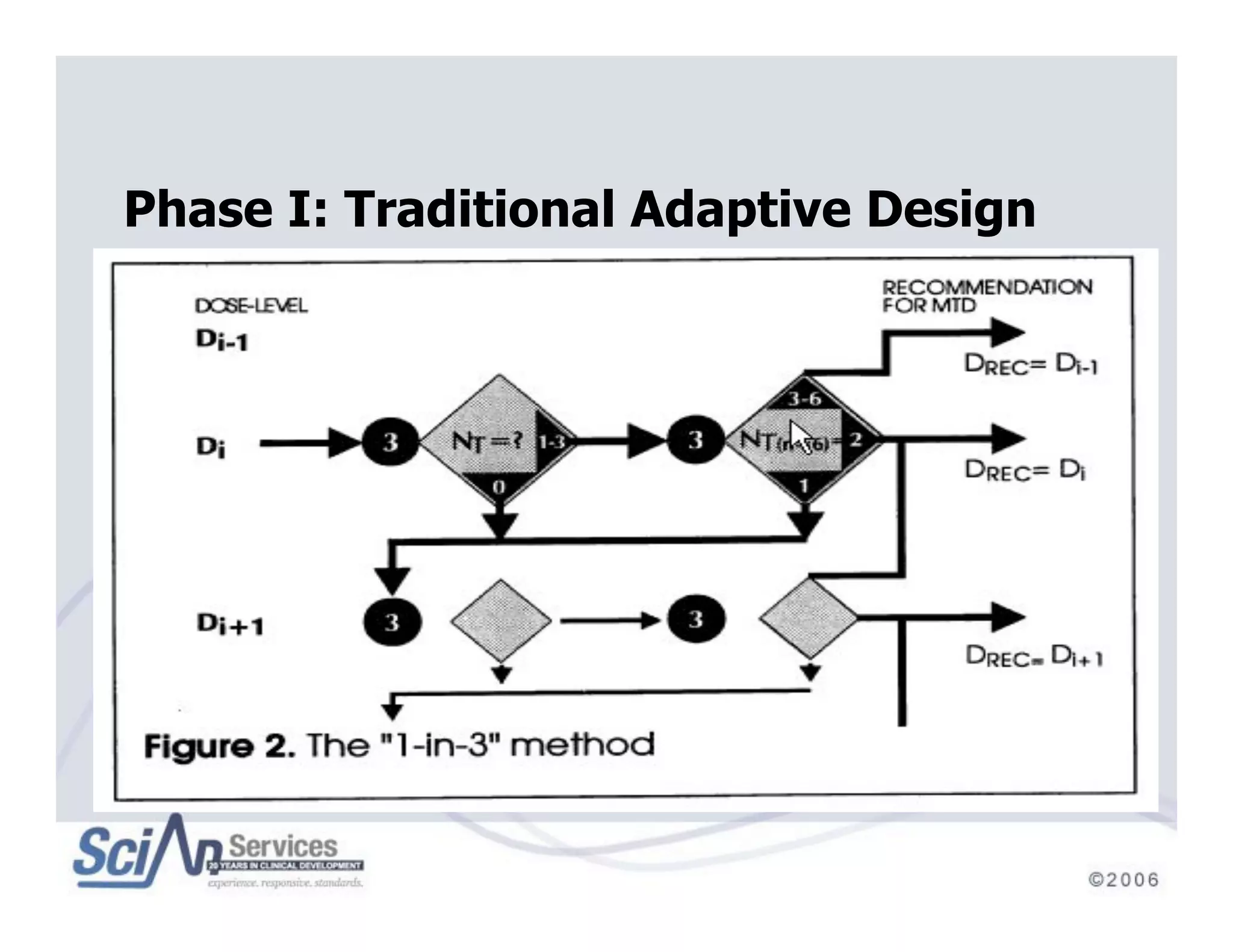
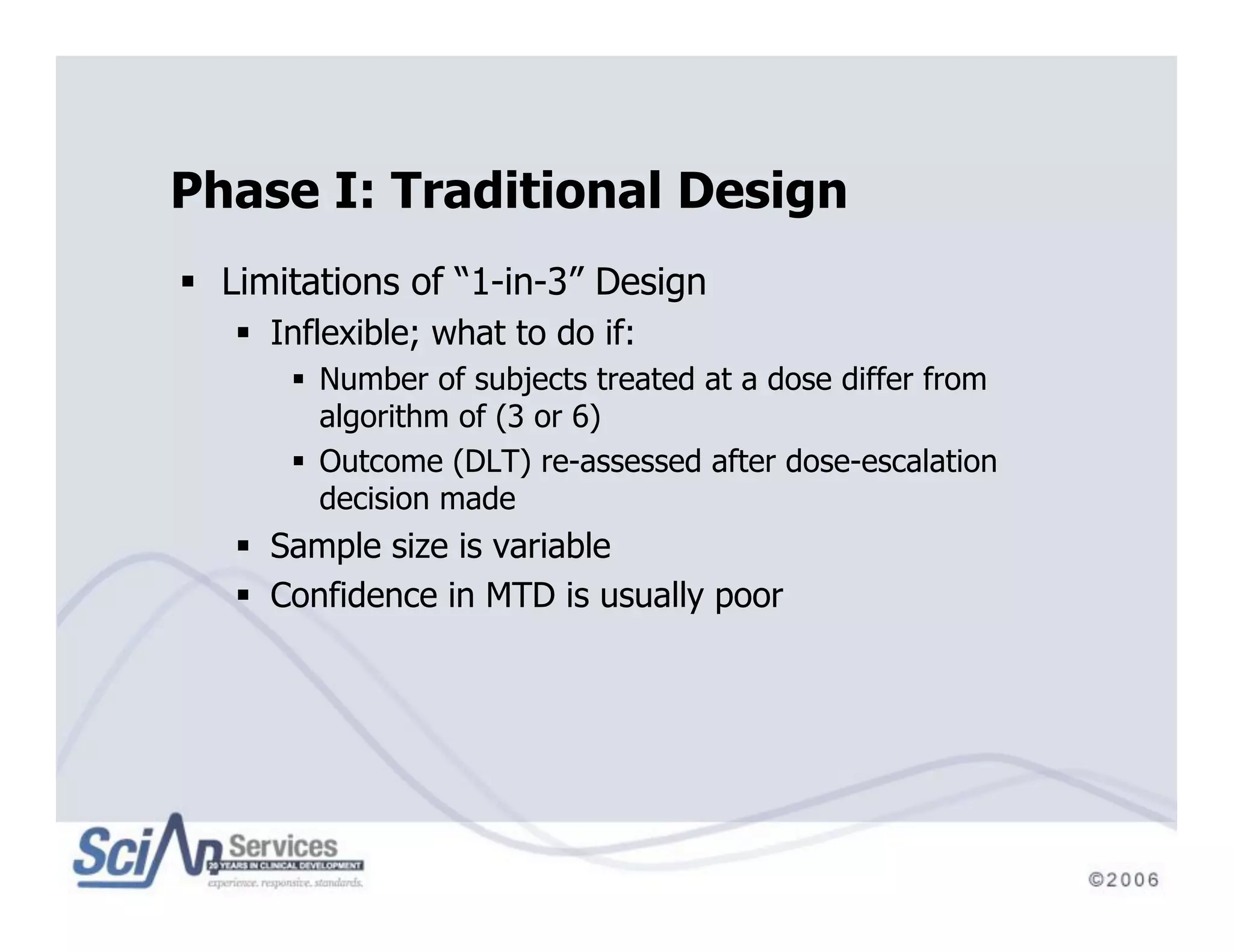
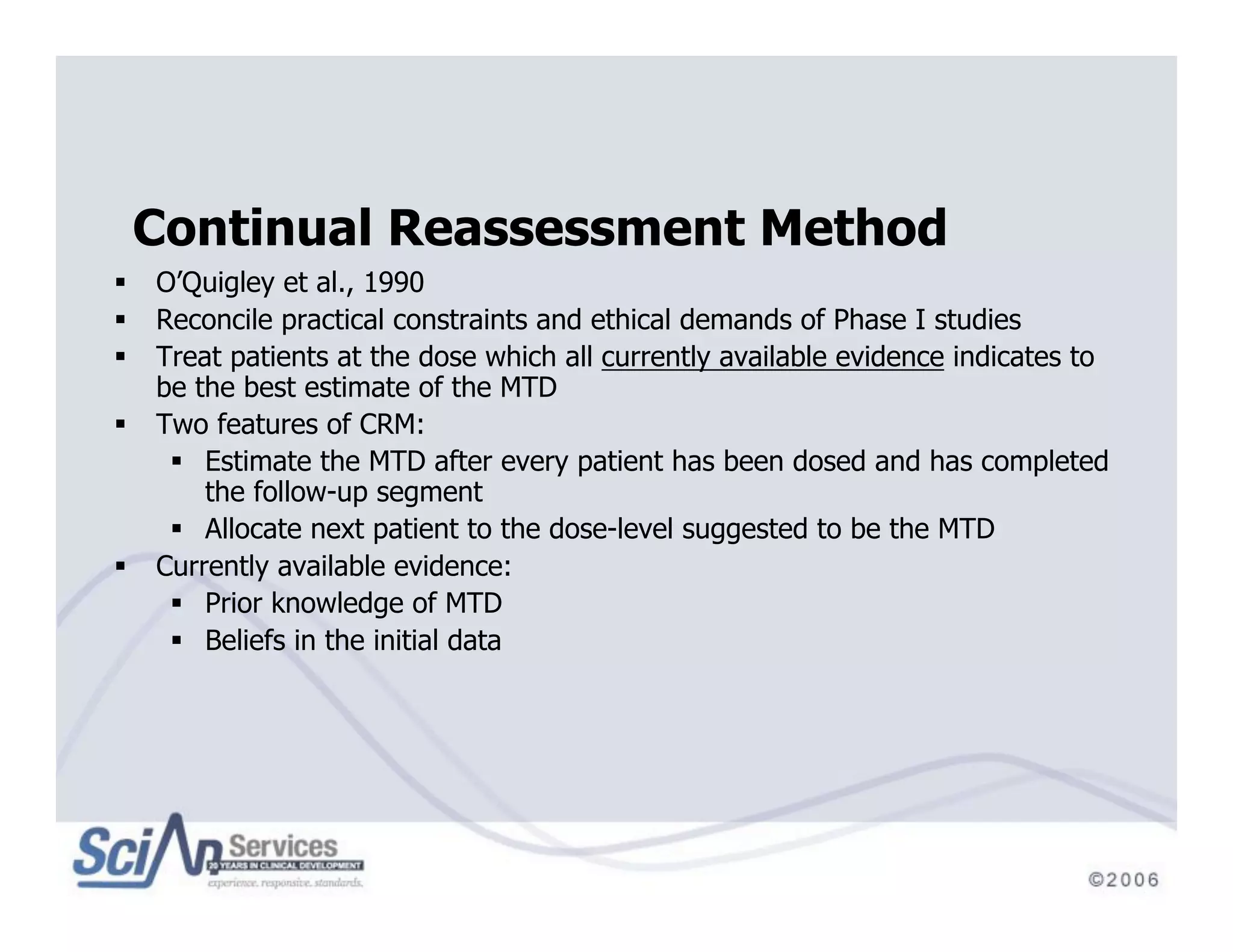
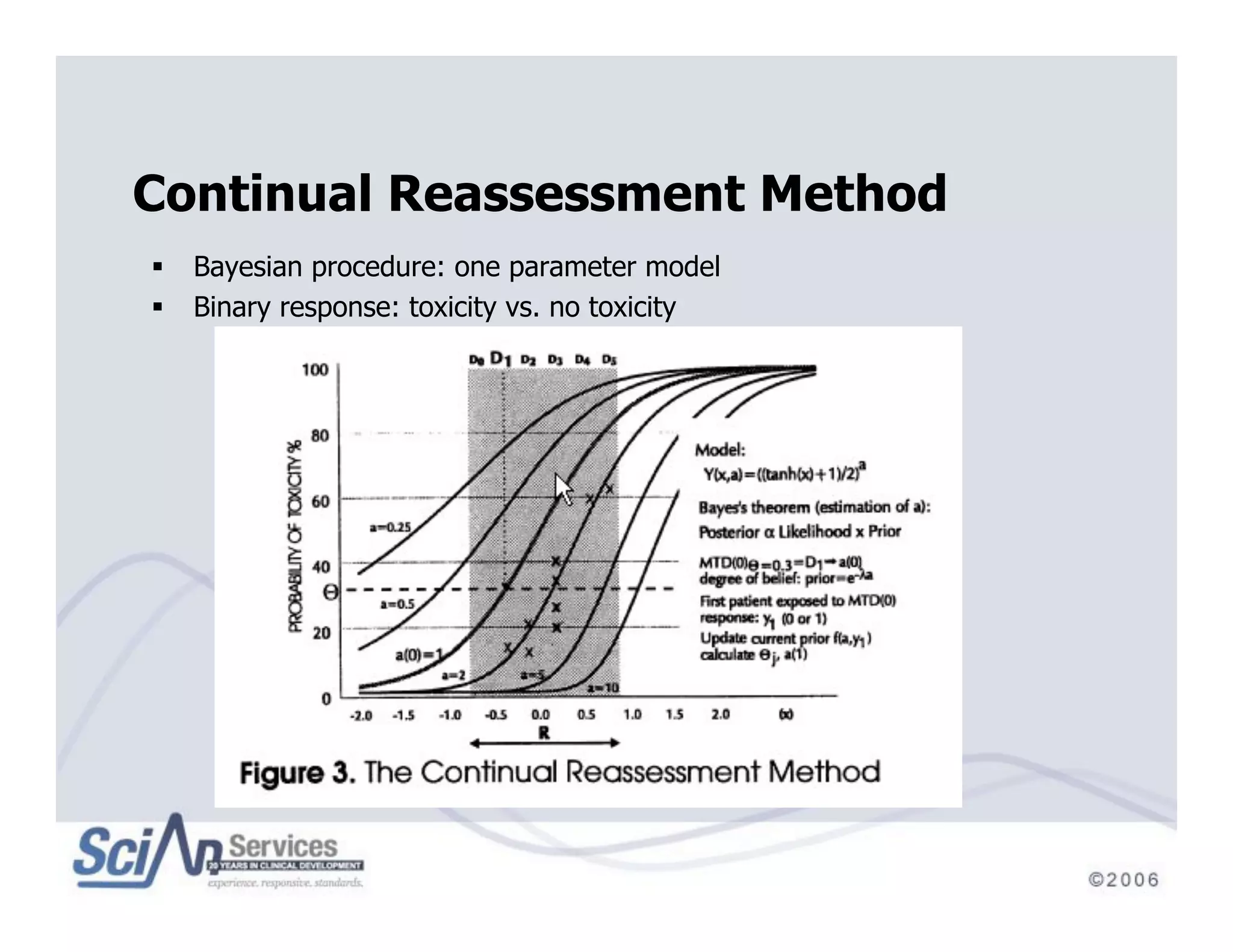
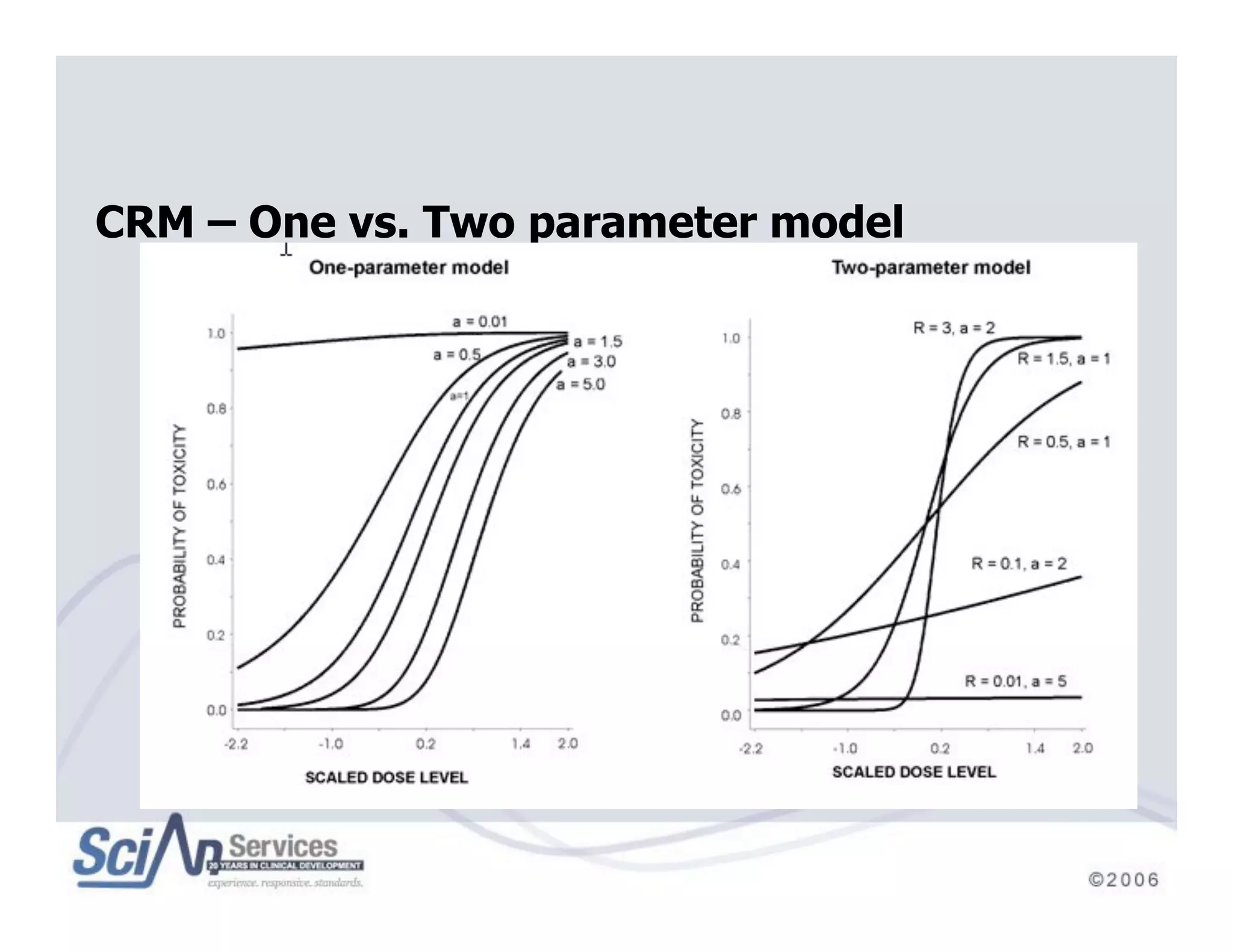
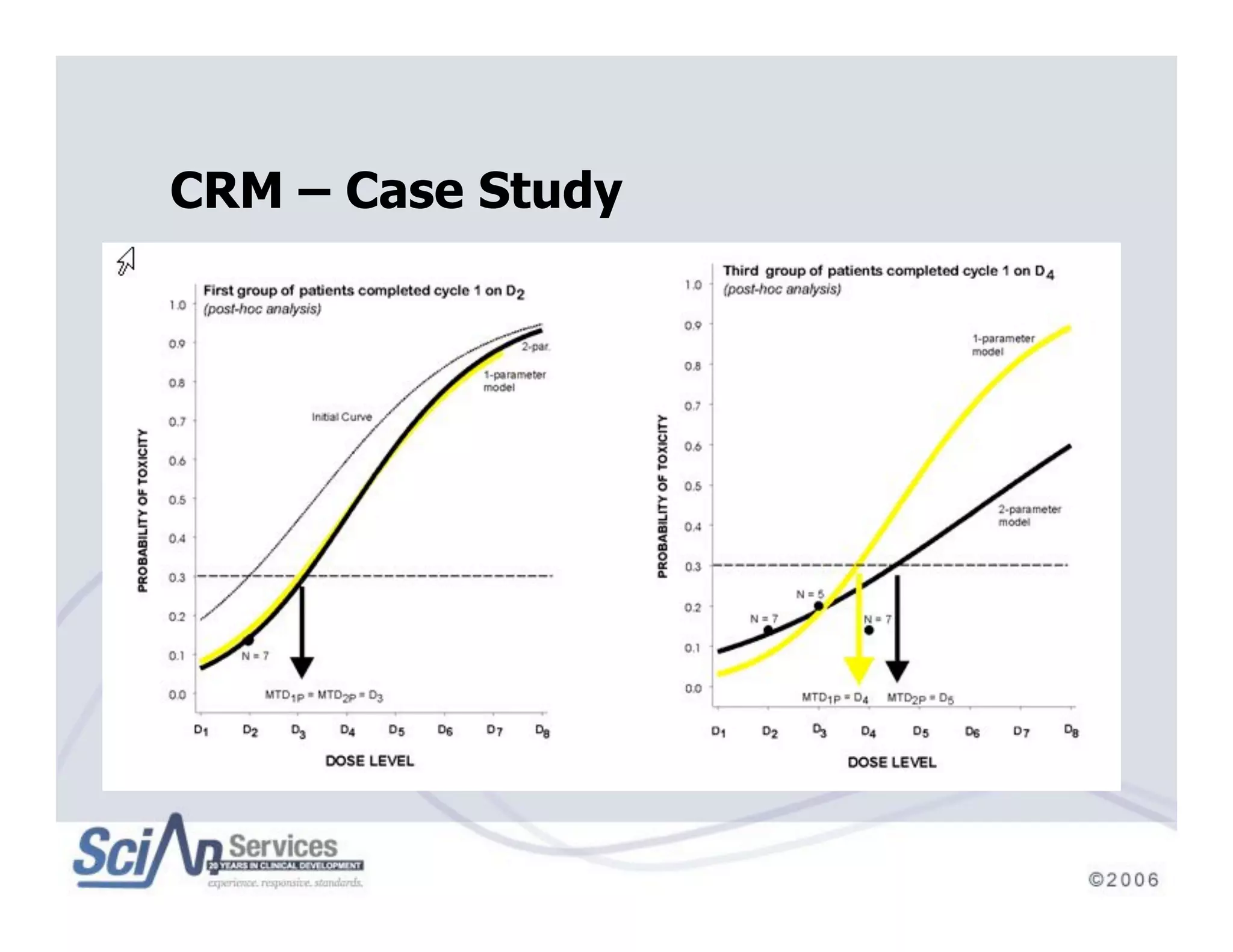
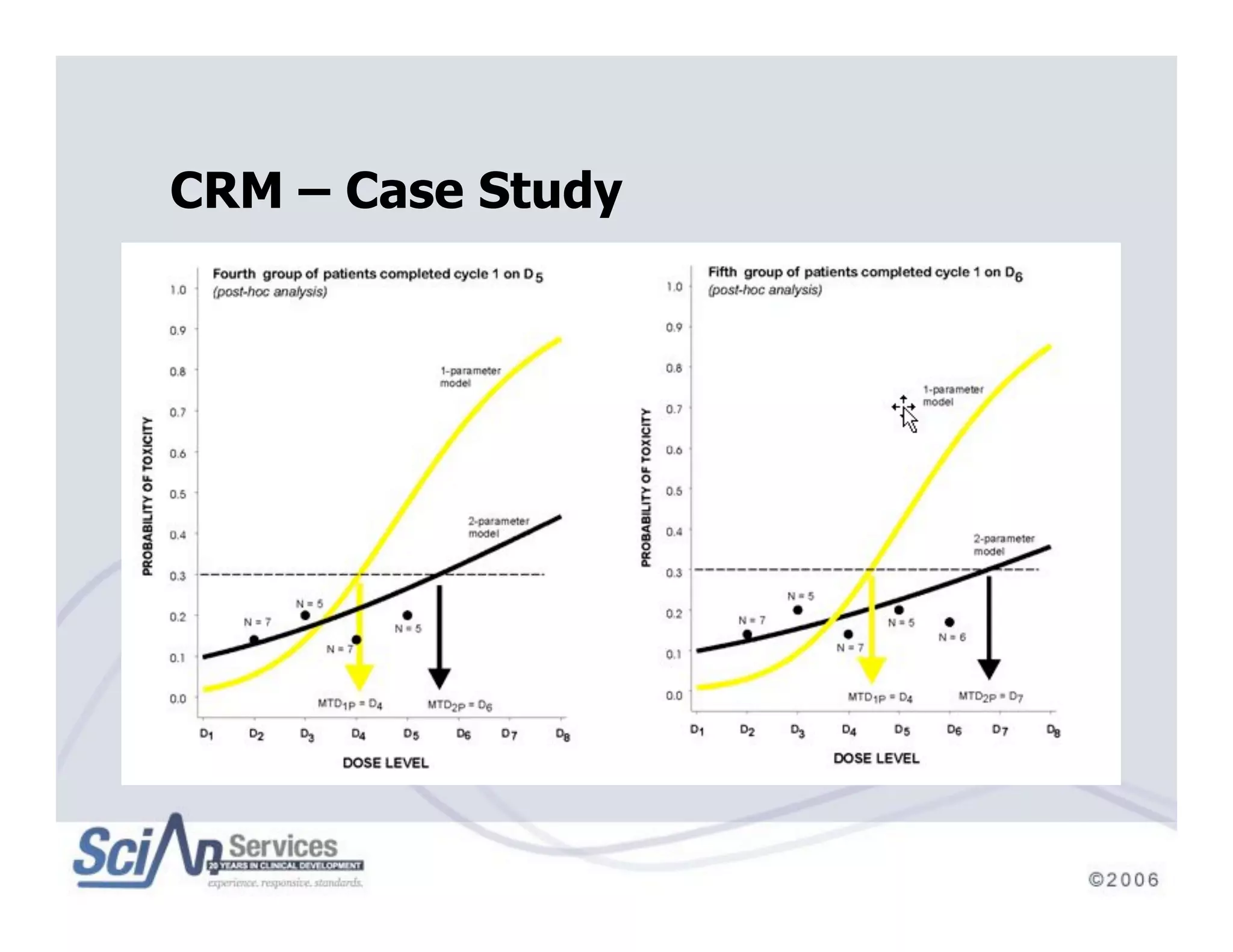








The document discusses advanced designs in early drug development, focusing on phase I trials and the limitations of traditional designs like the '1-in-3' method. It highlights the advantages of the Continual Reassessment Method (CRM) and its variants, demonstrating their efficacy in estimating the maximum tolerated dose (MTD) and addressing efficacy-toxicity trade-offs. Additionally, it emphasizes the need for Bayesian adaptive designs to enhance clinical trial efficiency and patient outcomes.























