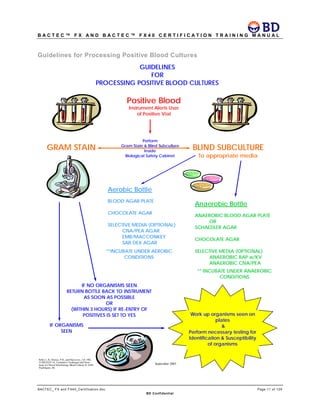
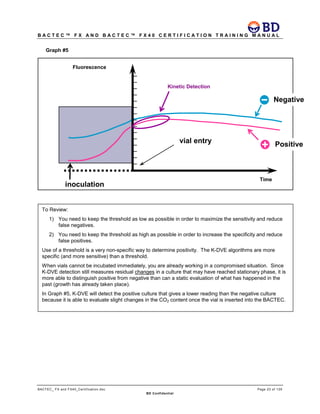
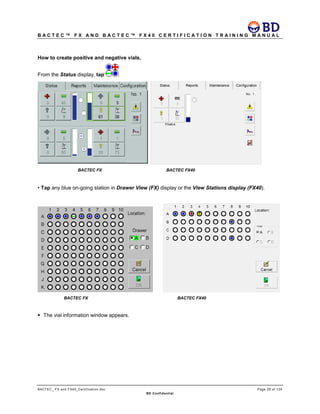
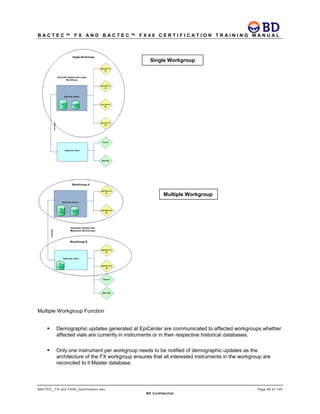
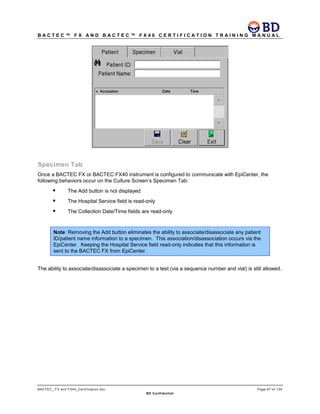
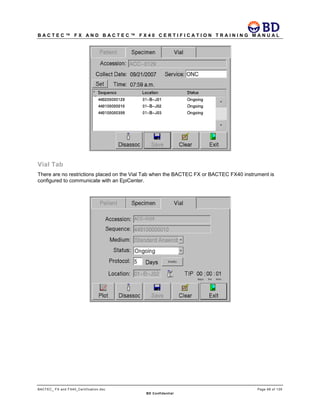
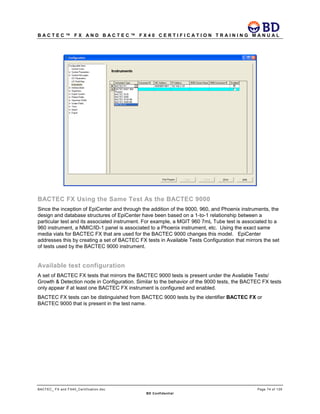
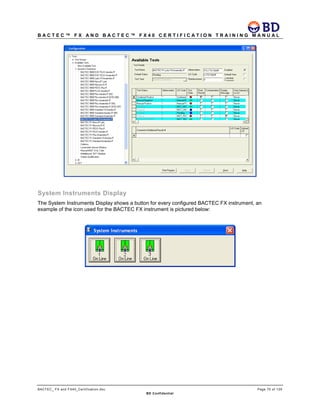
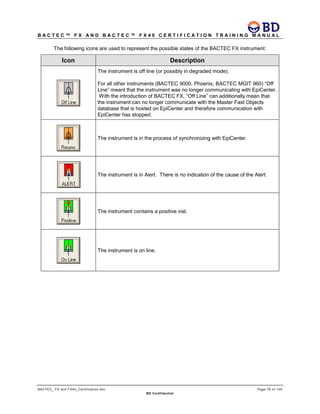
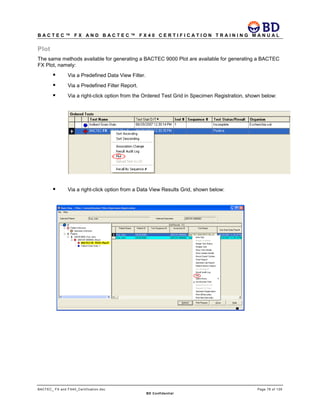
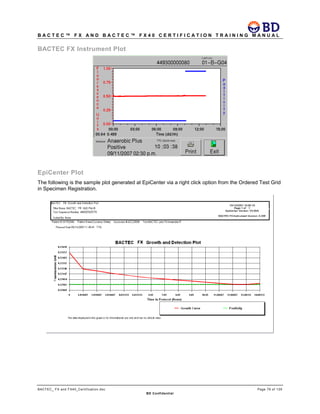
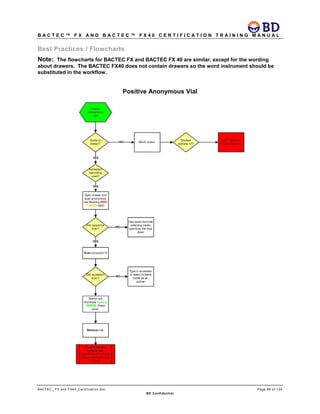
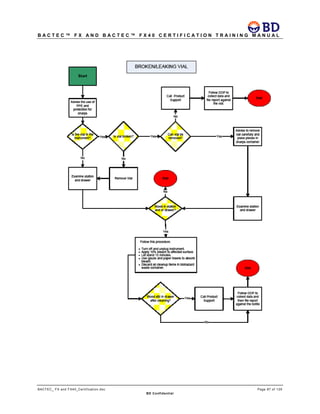
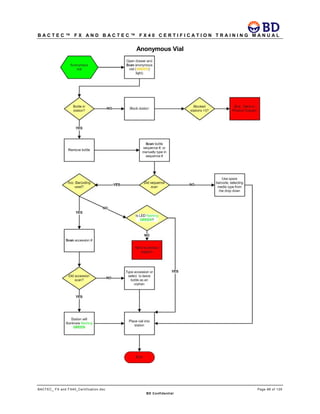
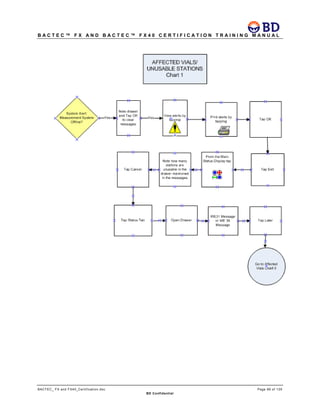
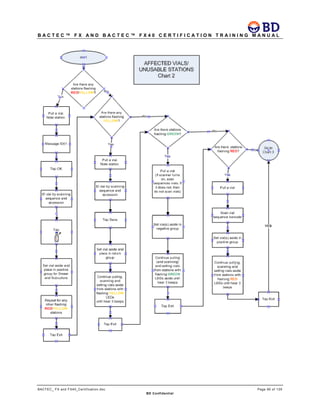
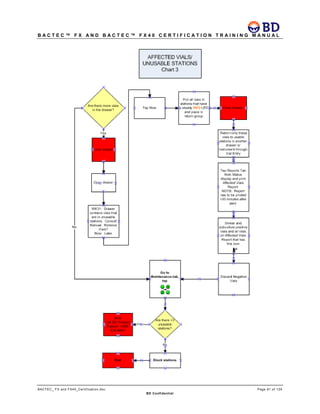
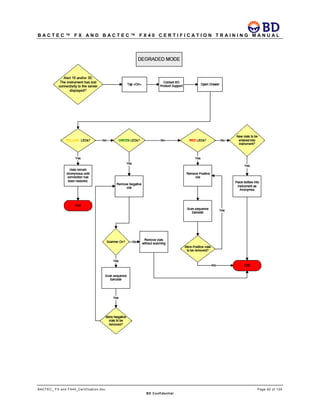
This document provides a certification training manual for BD BACTECTM FX and BD BACTECTM FX40 instruments. It outlines a three-tier training program to certify associates on the operation and maintenance of the instruments. Tier I provides basic operation training and certification, Tier II provides advanced training on instrument functionality and troubleshooting, and Tier III provides observation and support of on-site trainings. The training covers all aspects of using the instruments from specimen processing and vial entry to identifying positive cultures and maintaining the equipment. Successful completion of assessments is required to advance between tiers.