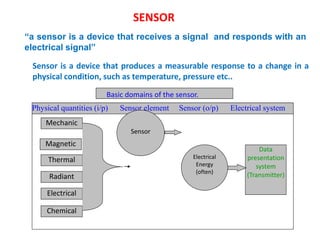
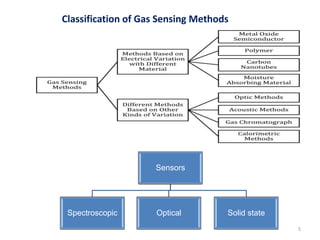
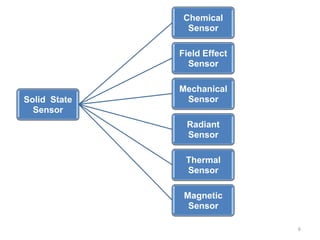
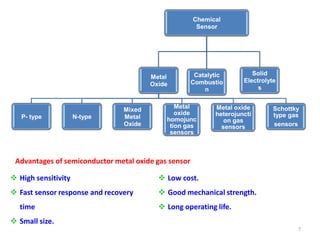
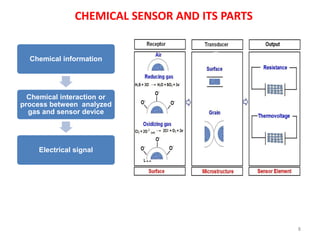
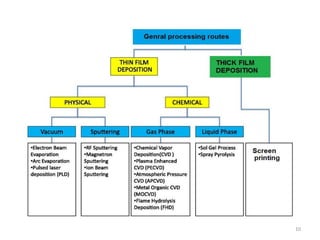
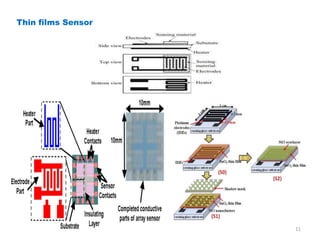
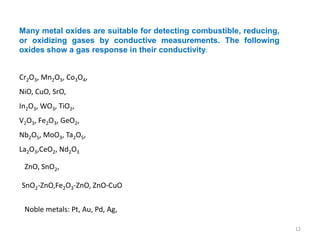
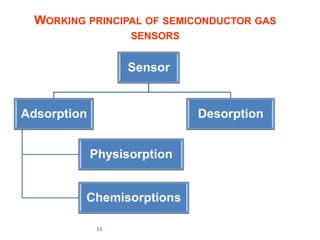
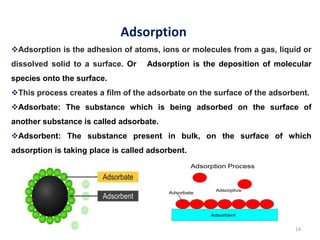
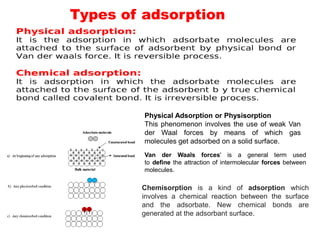
1. The document discusses thin film gas sensors and their operation. Thin film gas sensors use semiconductor metal oxides as the sensing material and operate by adsorption and desorption of gas molecules on the sensor surface.
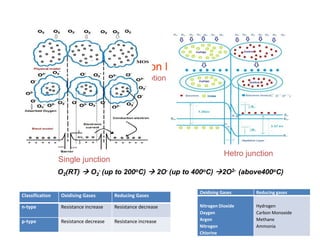
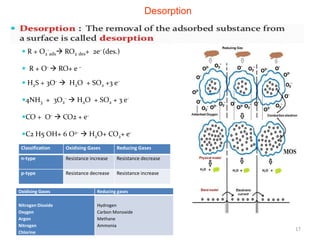
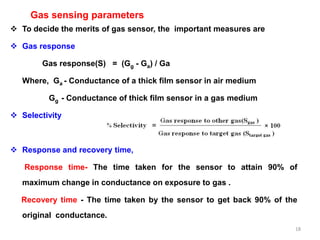
2. Gas detection is based on changes in the sensor's electrical conductivity from adsorption of gases. Oxidizing gases generally decrease resistance for n-type materials and increase resistance for p-type materials, while reducing gases have the opposite effects.
3. Key gases that can be detected include hydrogen, carbon monoxide, methane, and ammonia. Tin dioxide and zinc oxide are common thin film materials used. Characterization techniques like XRD and SEM are used to analyze the thin films and