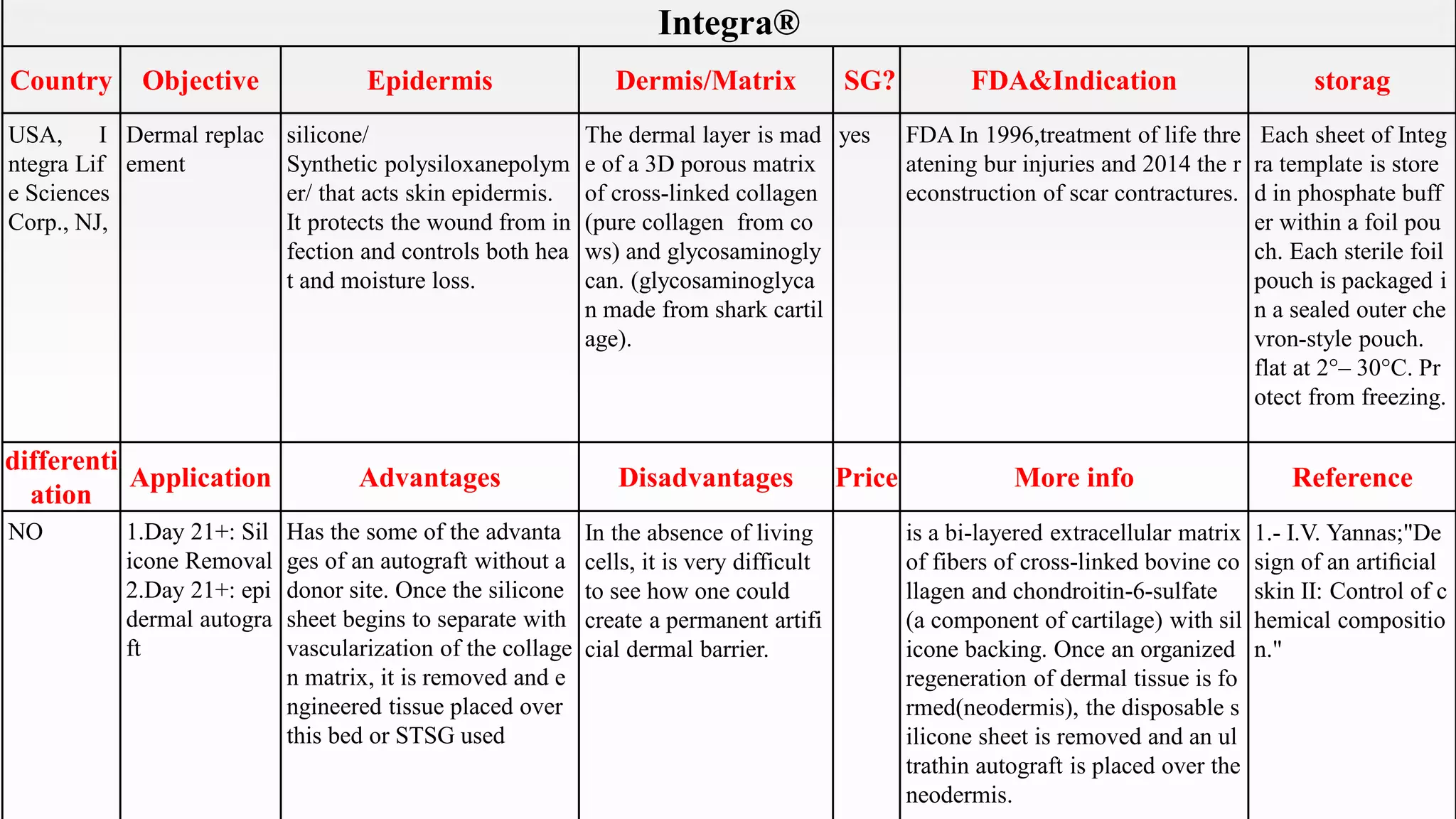
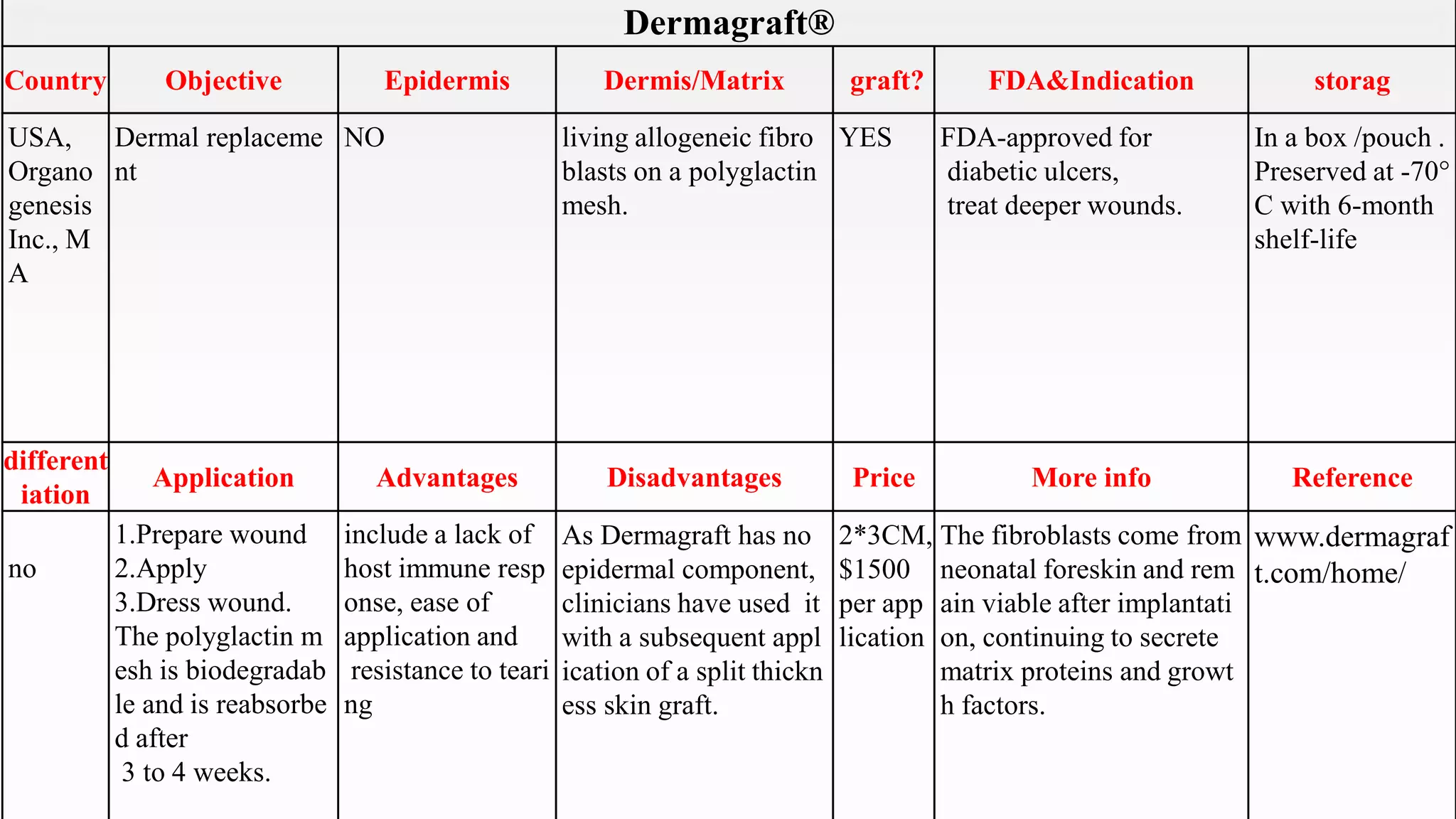
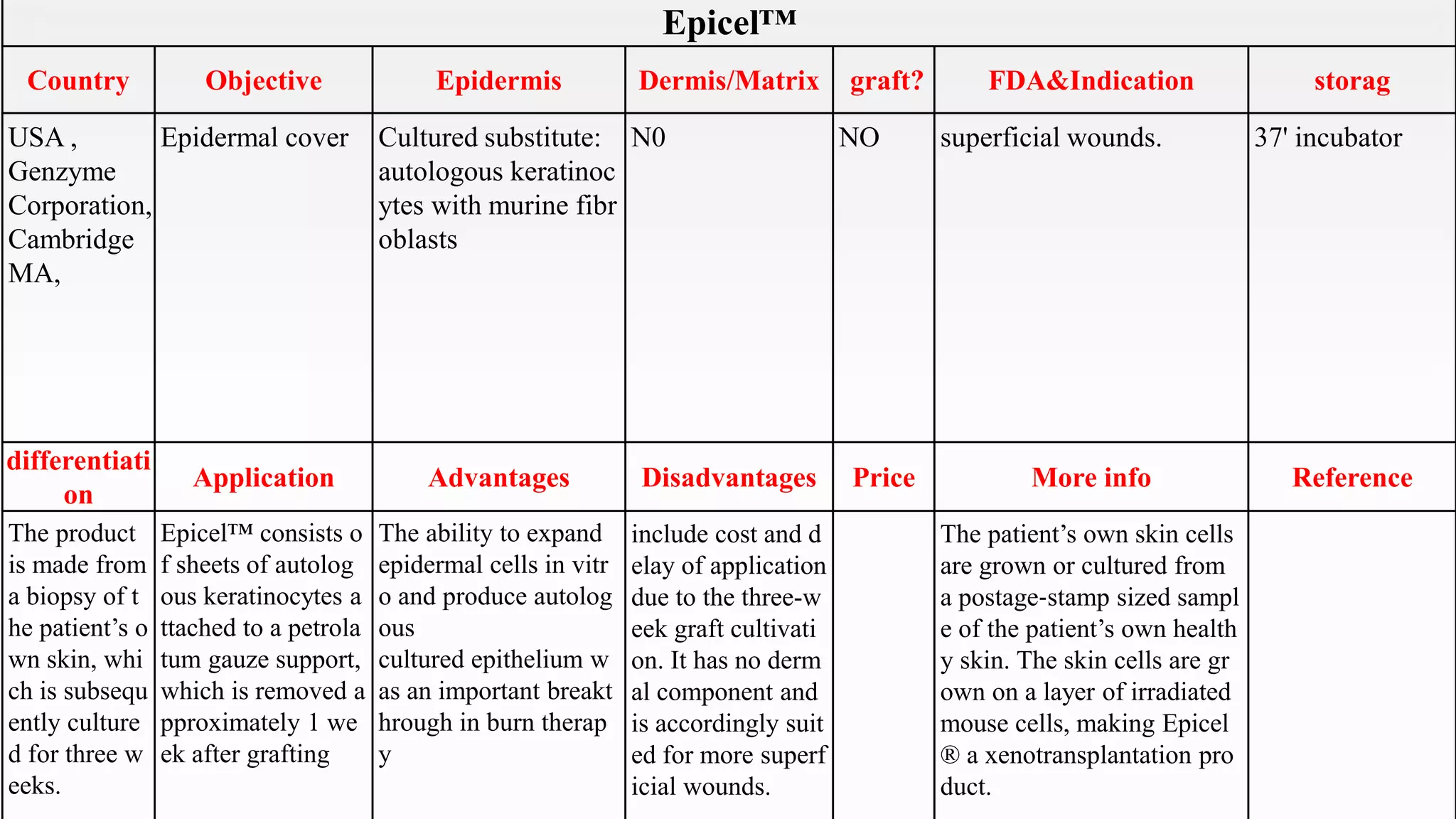
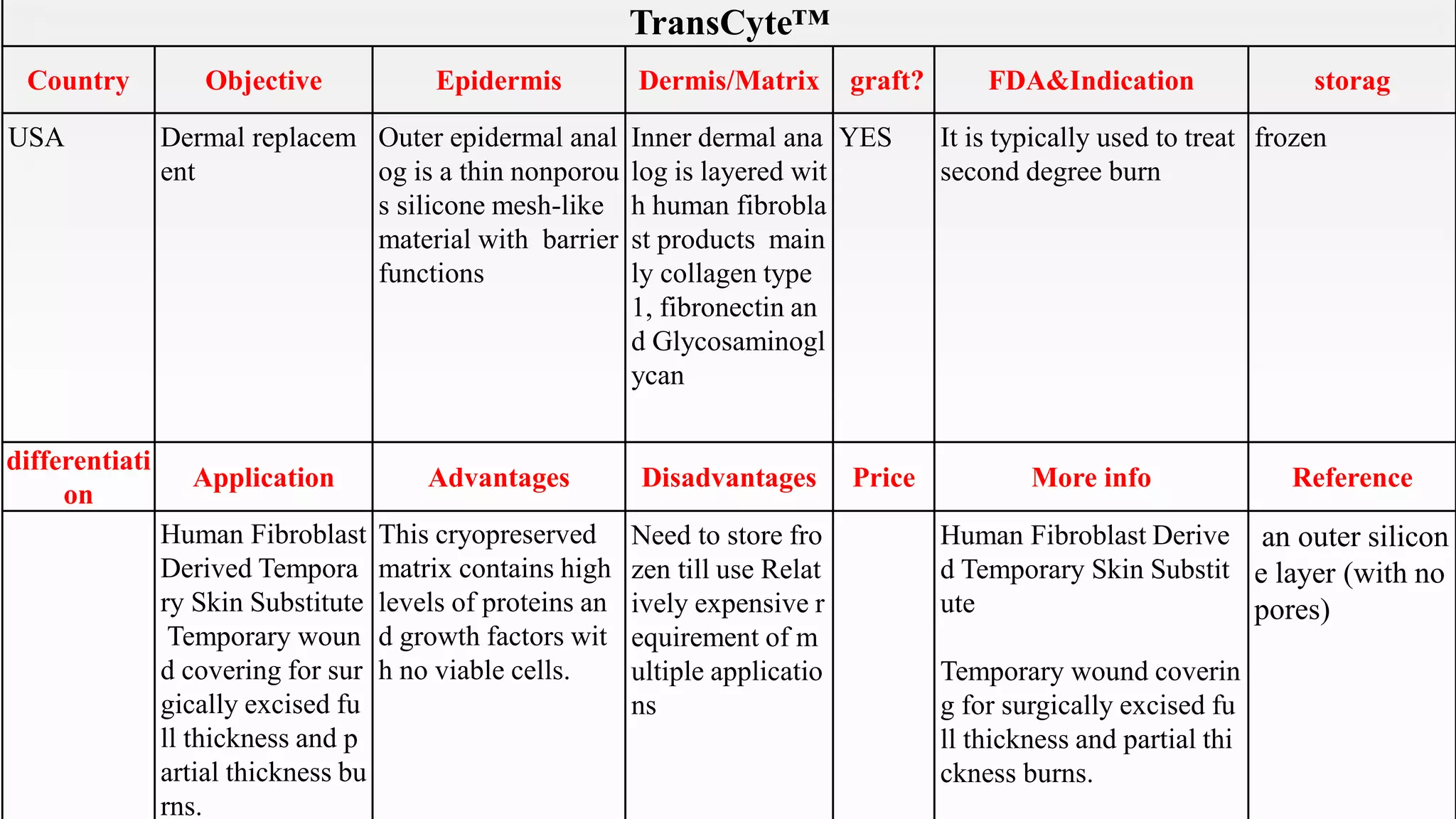
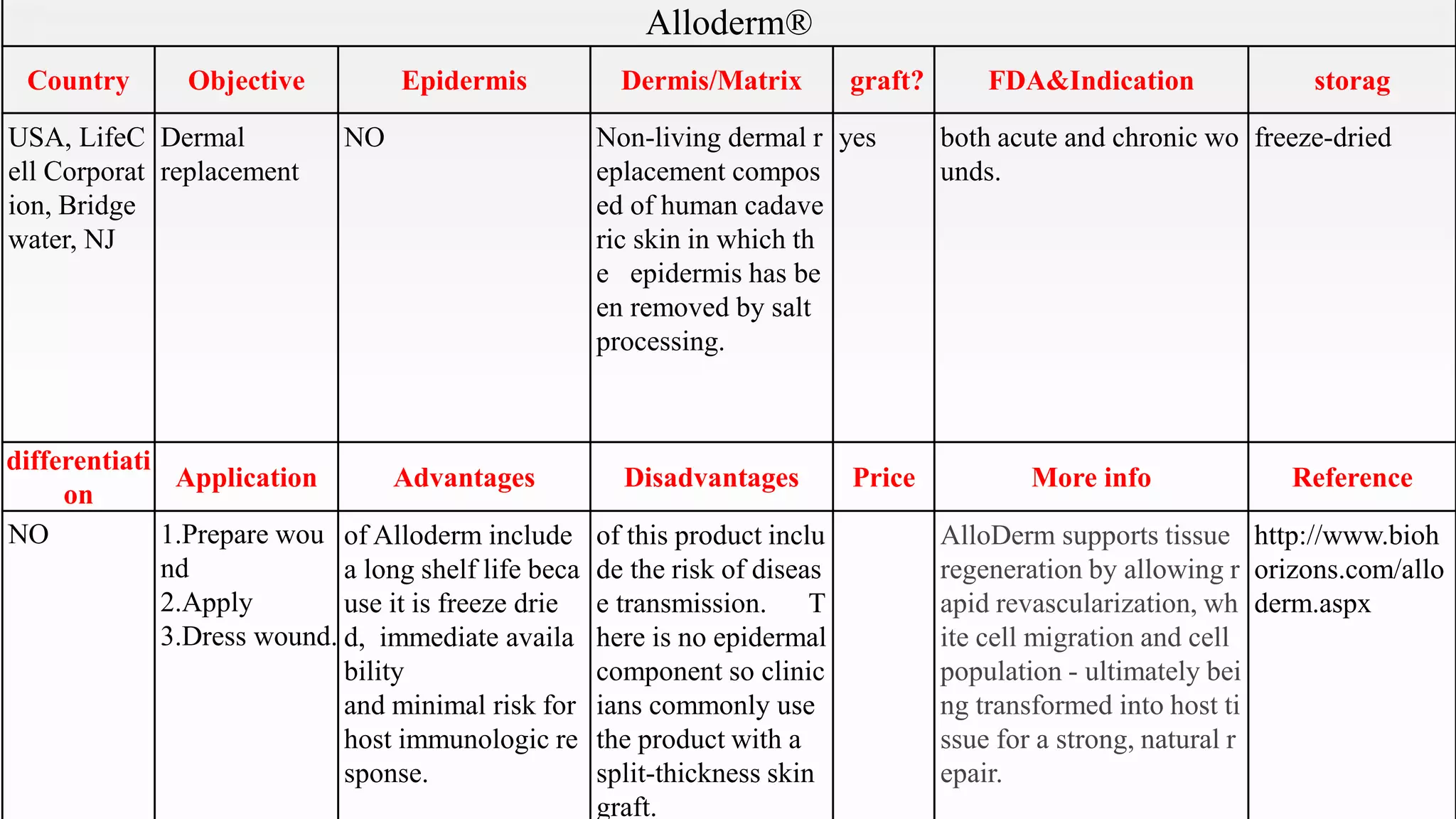
This document summarizes several artificial skin products:
- Apligraf, OrCel, Integra, Dermagraft, Epicel, TransCyte, Alloderm, Oasis, Matriderm, and Biobrane. It describes their objectives, components, FDA approvals, applications, advantages, disadvantages, pricing and additional information. The artificial skins are aimed at replacing skin layers for wound healing and skin grafts. They utilize various cell types, matrices, and synthetic or biological materials in their construction.