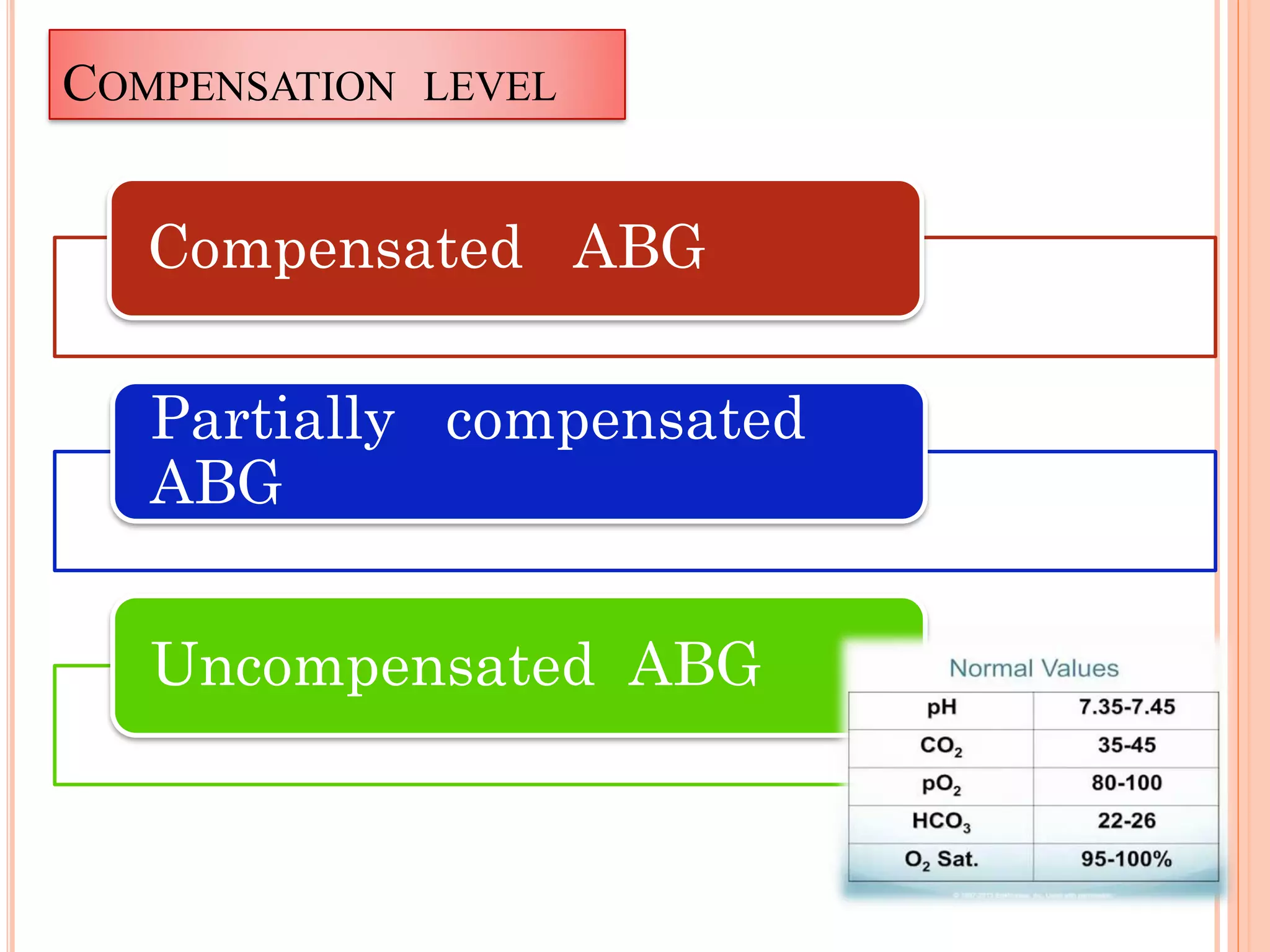
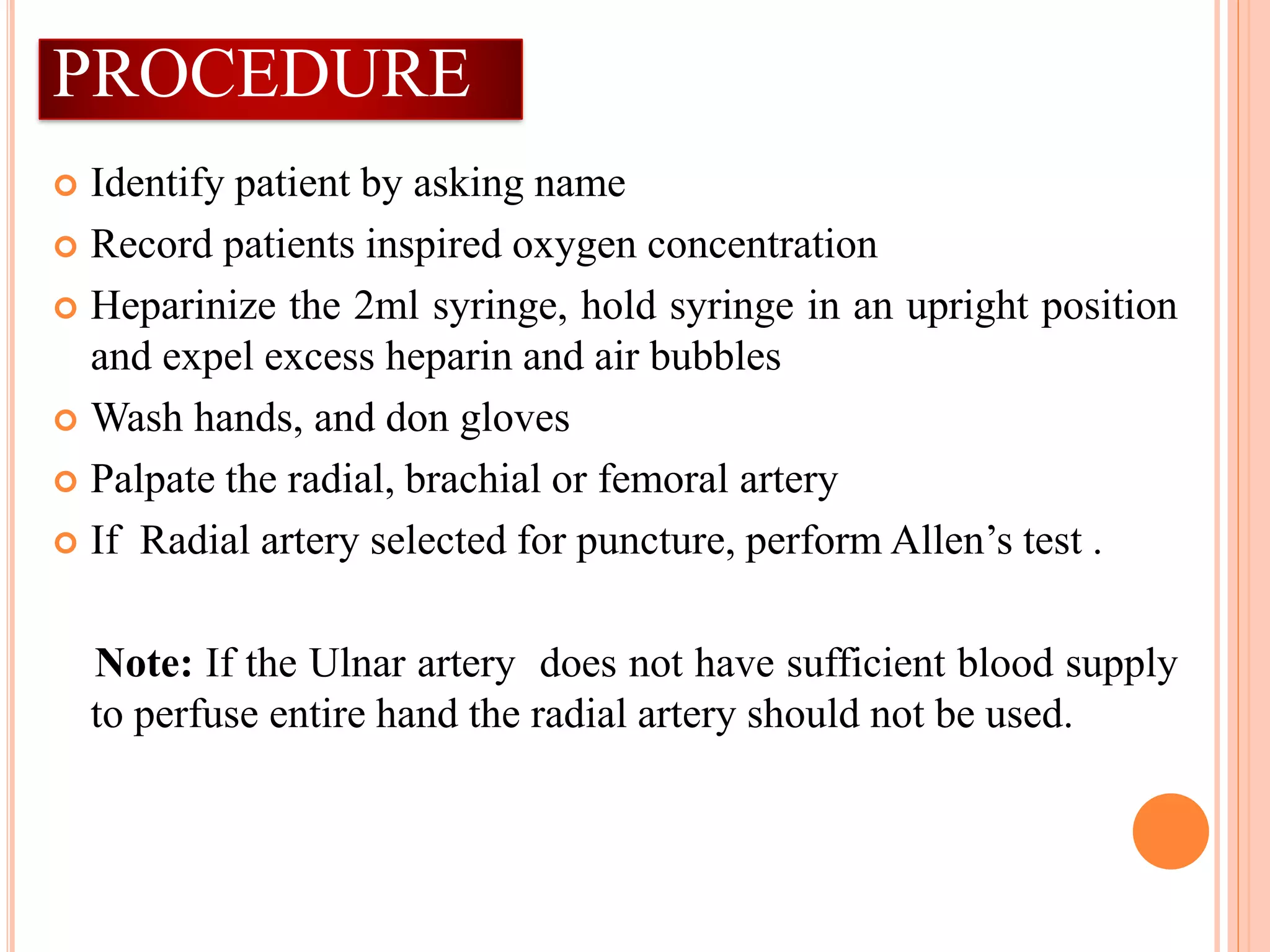
The document outlines the process and importance of arterial blood gas analysis, highlighting how the body maintains acid-base balance through various systems including the buffer, respiratory, and renal systems. It discusses the classifications and management of acid-base imbalances, such as respiratory and metabolic acidosis/alkalosis, along with indications and contraindications for arterial blood sampling. Additionally, it provides detailed procedural guidelines for performing arterial punctures and the requirements for analyzing blood gas specimens.
![ARTERIAL BLOOD GAS
ANALYSIS
Presented by
Mr. B.Kalyan kumar Msc[N]
Dept Of MSN](https://image.slidesharecdn.com/abgarterialbloodgasanalysis-converted-200424074017/75/Arterial-blood-gas-analysis-ABG-1-2048.jpg)