Embed presentation
Download to read offline












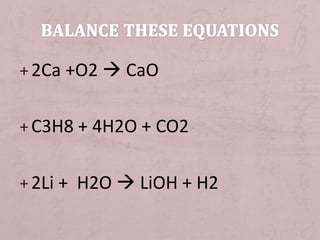
The document discusses the steps for writing and balancing chemical equations. It explains that reactants are identified and written on one side of the equation, and products are written on the other side. Chemical symbols are used to write unbalanced equations, and the numbers of atoms are adjusted to balance the equation so there are the same number of each type of atom on both sides. Signs that a reaction occurred include the release or absorption of energy as heat or light.












