This document summarizes key concepts about ions and ionic compounds from a chemistry chapter:
1) Atoms form ions by gaining or losing electrons to achieve a stable electron configuration like neon's. Metals tend to lose electrons to form cations while nonmetals gain electrons to form anions.
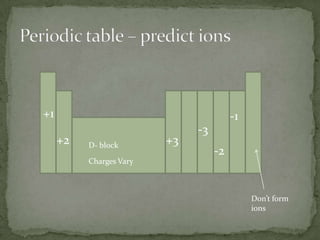
2) Using the periodic table, one can determine an atom's valence electrons and predict what stable ions it will form by gaining or losing electrons to achieve an octet. For example, potassium loses one electron to form the K+ ion.
3) Ions have very different properties from their parent atoms. For instance, sodium and chlorine are reactive as atoms but form the stable ionic compound table salt

![Objective 1:Relate the electron configuration of an atom to its chemical reactivity.Chemical ReactivityDepends on achievement of octet rules2p6 electron configuration - eight valence electrons[O] s2p4 Needs 2 electrons[Ne] s2p6 Needs 0 electrons – stableMost Reactive:Alkali Metals [Na] s1 Needs to lose 1 electronHalogen [Cl] s2p5 Needs 1 electron](https://image.slidesharecdn.com/chapter5-1applied-100331135621-phpapp02/85/Applied-Chapter-5-1-Simple-Ions-2-320.jpg)
![Objective 2Determine an atom’s number of valence electrons, and use the octet rule to predict what stable ions the atom is likely to form.Valence ElectronsUse periodic table – electron configurationEx: [Mg] s22 valence electrons [P] s2p35 valence electronIon – atoms that has gained or lost one or more electrons and has a positive or negative charge](https://image.slidesharecdn.com/chapter5-1applied-100331135621-phpapp02/85/Applied-Chapter-5-1-Simple-Ions-3-320.jpg)
![Forming a Cation – positive charge[K] s1 will lose 1 electron Creating +1 ionSo K+ is formed. Called a potassium ionSTABLEForming an Anion – Negative charge[S] s2p4 will gain 2 electrons Creating -2 ionSo S-2 is formed. Called a sulfur ionSTABLEBoth Now have s2p6 configuration](https://image.slidesharecdn.com/chapter5-1applied-100331135621-phpapp02/85/Applied-Chapter-5-1-Simple-Ions-4-320.jpg)