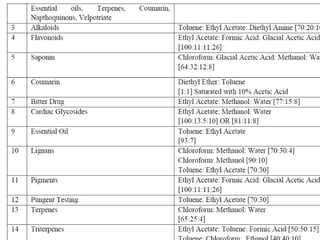
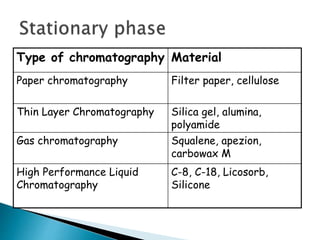
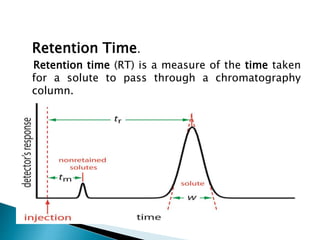
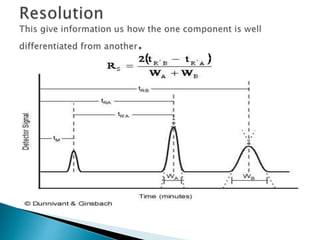
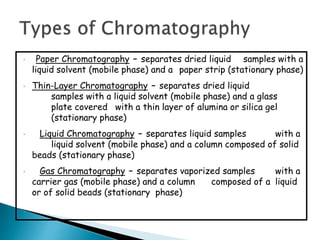
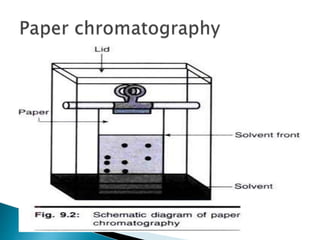
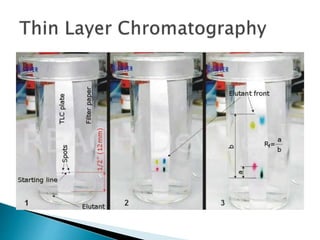
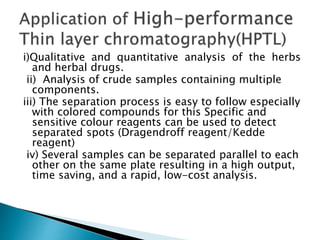
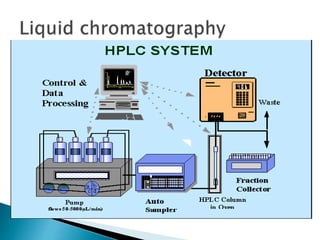
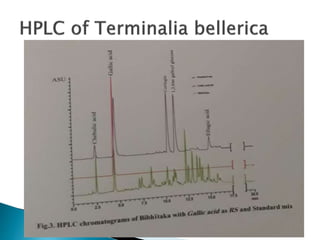
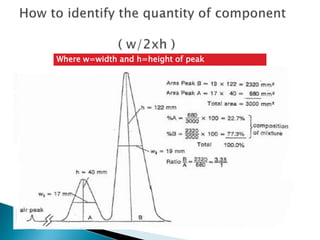
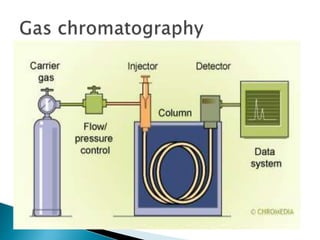
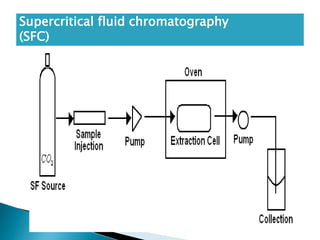
Chromatography is a technique used to separate components of a mixture using a mobile and stationary phase. The document discusses various chromatography techniques including paper chromatography, thin layer chromatography, liquid chromatography, gas chromatography, and supercritical fluid chromatography. It also discusses their applications in herbal drug analysis, standardization, and authentication which are important in fields like pharmaceuticals, forensics, and food production. Chromatography plays a key role in the identification, quantification, and quality control of herbal drugs and formulations in Ayurveda.