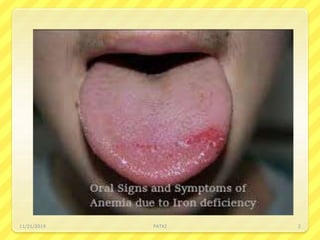
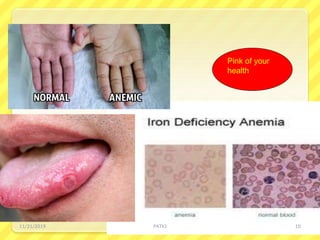
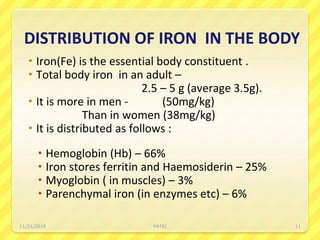
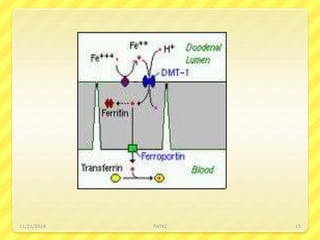
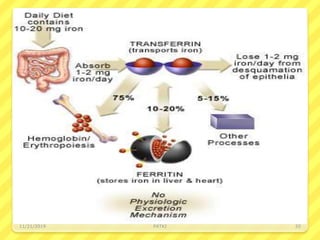
Iron deficiency anemia is the most common type of anemia seen in clinical practice. It is characterized by a decrease in hemoglobin and oxygen-carrying capacity due to low iron levels. Oral iron supplements are usually the first line treatment, with ferrous sulfate being a commonly used and inexpensive option. Parenteral iron is considered when oral iron is not tolerated or absorbed. The document provides details on causes of iron deficiency anemia, distribution and absorption of iron in the body, classification of anemias, oral and parenteral iron preparations and their administration, and indications and adverse effects of iron therapy.