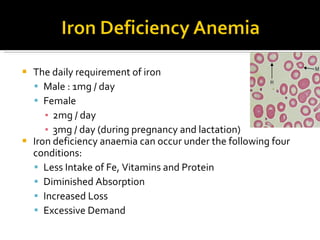
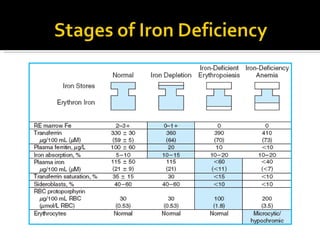
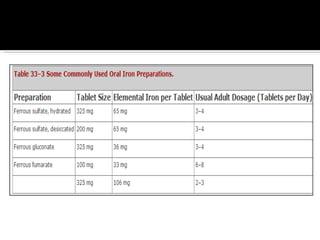
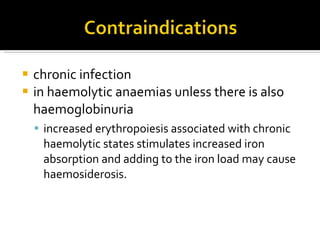
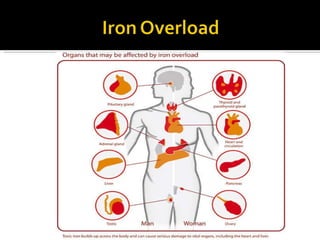
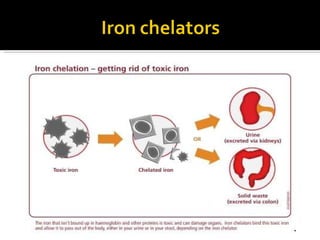
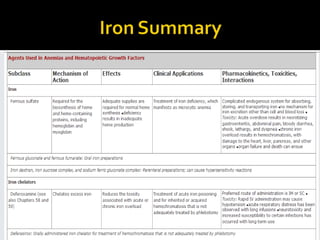
The document discusses hematinic agents such as iron, folic acid, and vitamin B12 which are used to treat anemia. It covers the pharmacokinetics of iron absorption and transport, indications for hematinic agents including iron deficiency anemia, drug interactions, side effects, and iron toxicity treatment with chelating agents.



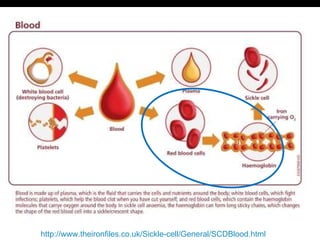
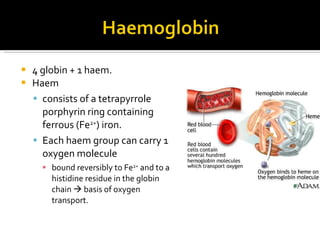
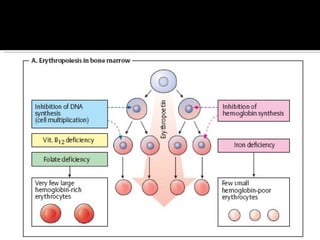
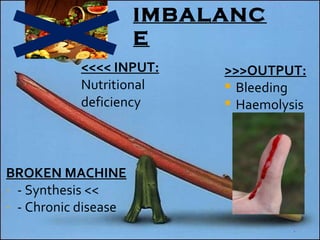
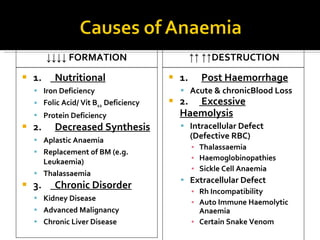
![Definition: ↓ [Hb] i n blood & / RBC per age, sex and geographical location. Normal Hb : 14g to 16g /dl in Male 13g to 15g /dl in Female Acute: fatigue chronic : asymptomatic. Classification based on indices of red cell are : hypochromic, microcytic anaemia macrocytic anaemia normochromic normocytic anaemia mixed pictures.](https://image.slidesharecdn.com/hematiniciarf-12799806337357-phpapp01/85/Hematinic-I-7-320.jpg)