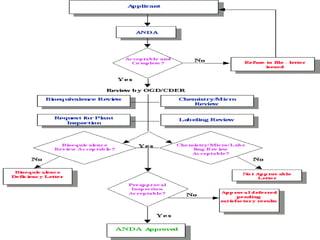
The ANDA regulatory approval process involves an applicant submitting an ANDA to the FDA's Office of Generic Drugs. The OGD ensures generic drugs are safe and effective through a review process similar to NDAs. The ANDA review process involves filling review, coordination of review, bioequivalence review, chemistry review, labeling review, and final approval. Once approved, the applicant can manufacture and market the generic drug as a lower-cost alternative. The primary difference from NDAs is generic drugs must demonstrate bioequivalence to the reference listed drug but do not require new clinical trials.