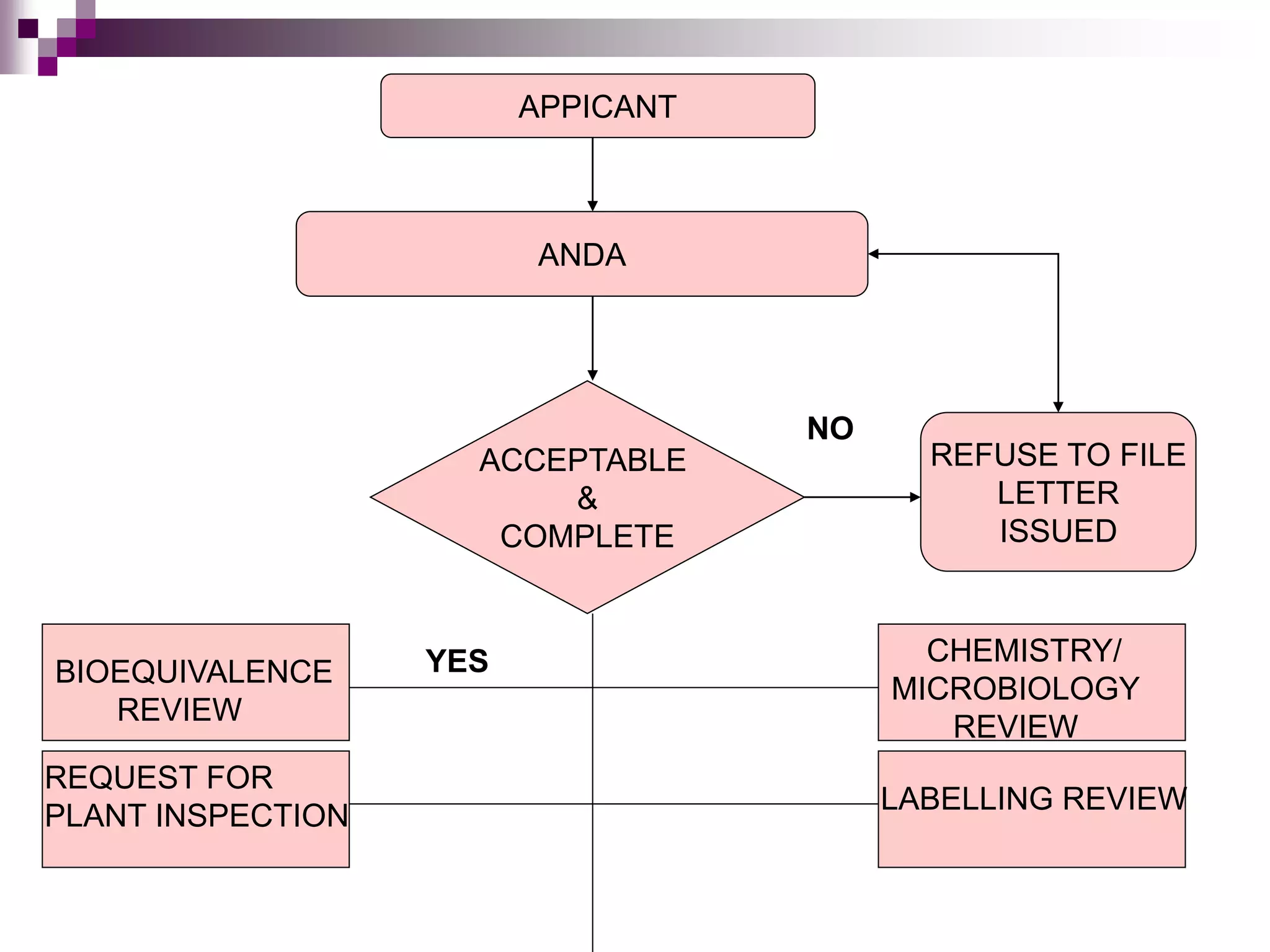
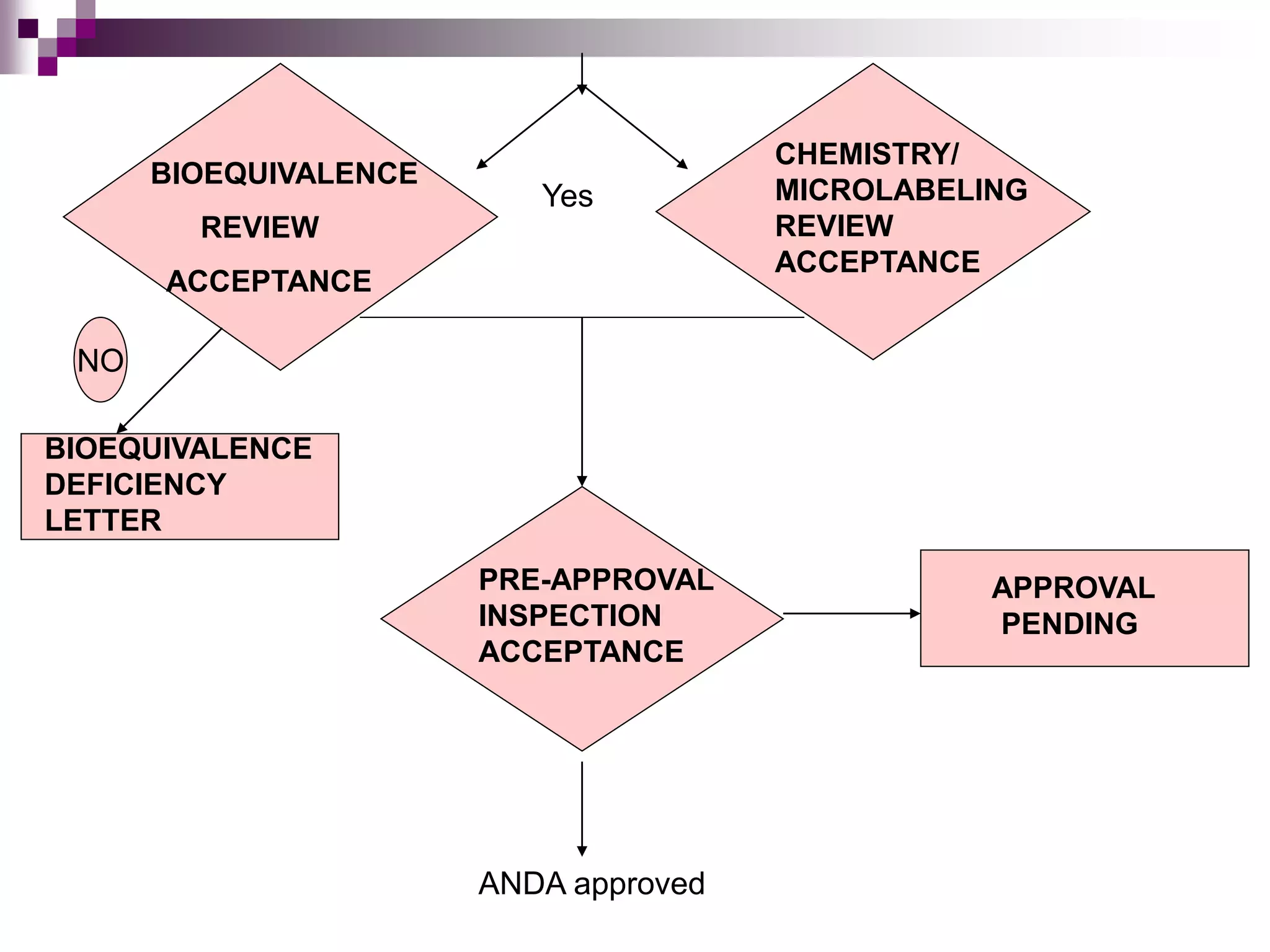
An Abbreviated New Drug Application (ANDA) contains data to allow the FDA to review and approve a generic drug. The ANDA process provides a way for generic drug applications to be approved without requiring clinical trials by demonstrating bioequivalence to an existing brand name drug. The FDA reviews the ANDA for bioequivalence, chemistry and manufacturing controls, labeling, and conducts inspections to ensure quality before final approval of a generic drug.