Analeptics are central nervous system stimulants used for conditions such as depression and respiratory depression, including drugs like doxapram and pentylene tetrazole. Doxapram acts as a respiratory stimulant by stimulating the medulla, while pentylene tetrazole blocks GABA receptor function and has convulsant properties. Both are primarily utilized in medical settings to aid in anesthetic recovery and treat emergency respiratory issues.






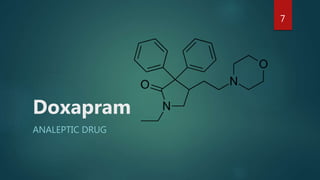



![Synthesis- Doxapram
Doxapram marketed and medically used as racemate, has a stereogenic
centre.
it possess [+] enantiomer and [-] enantiomer
[+] enantiomer possess pharmacological activity while [-] enantiomer is
inactive.
The doxapram can be prepared by multiple crystallization of
dibenzoyltartaric acid salts. But loss of material during crystallization is
unsuitable in industrial scale.
11
Dibenzoyltartaric acid salt](https://image.slidesharecdn.com/analeptics-200610170610/85/ANALEPTICS-DOXAPRAM-11-320.jpg)
















