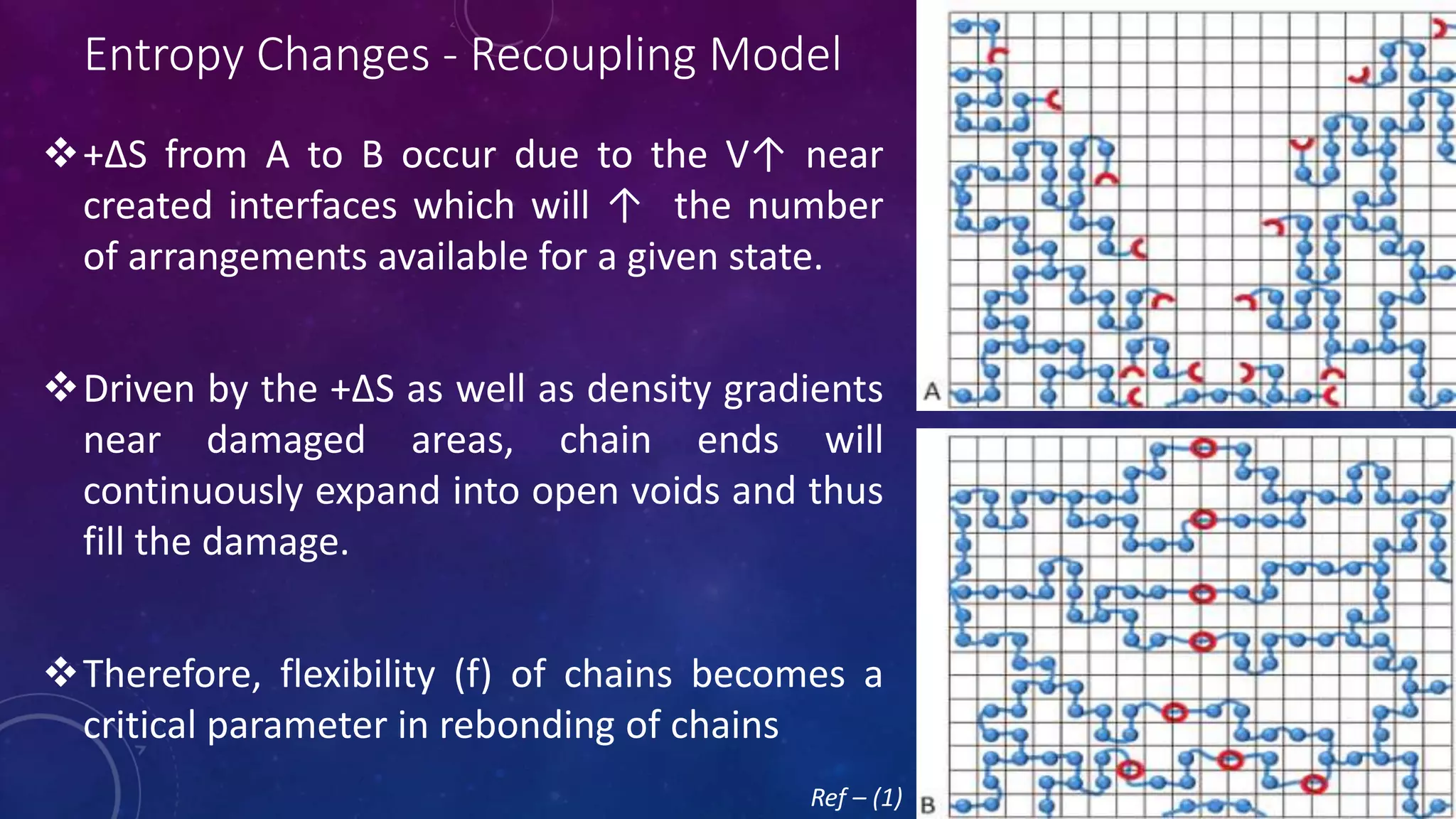
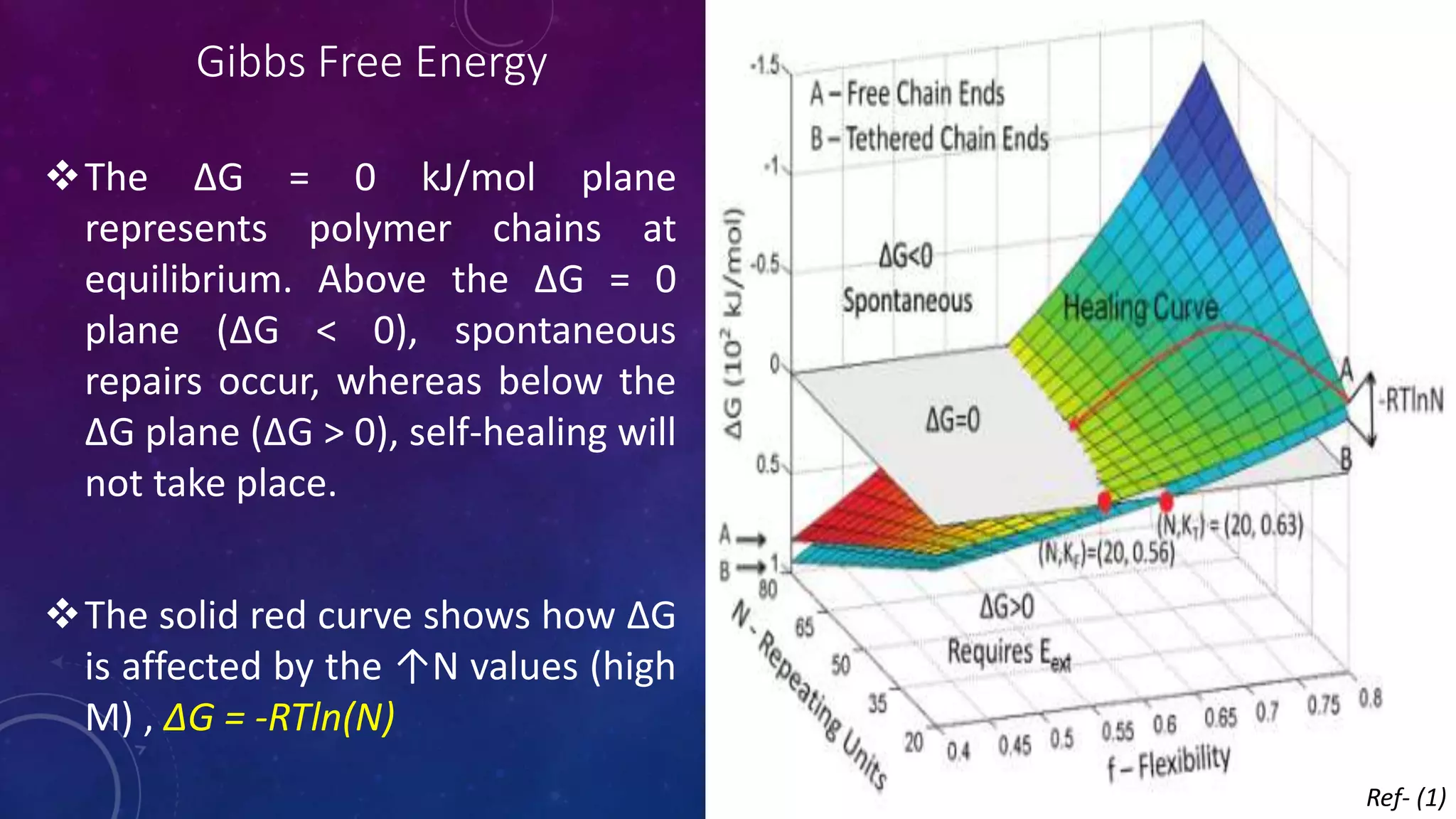
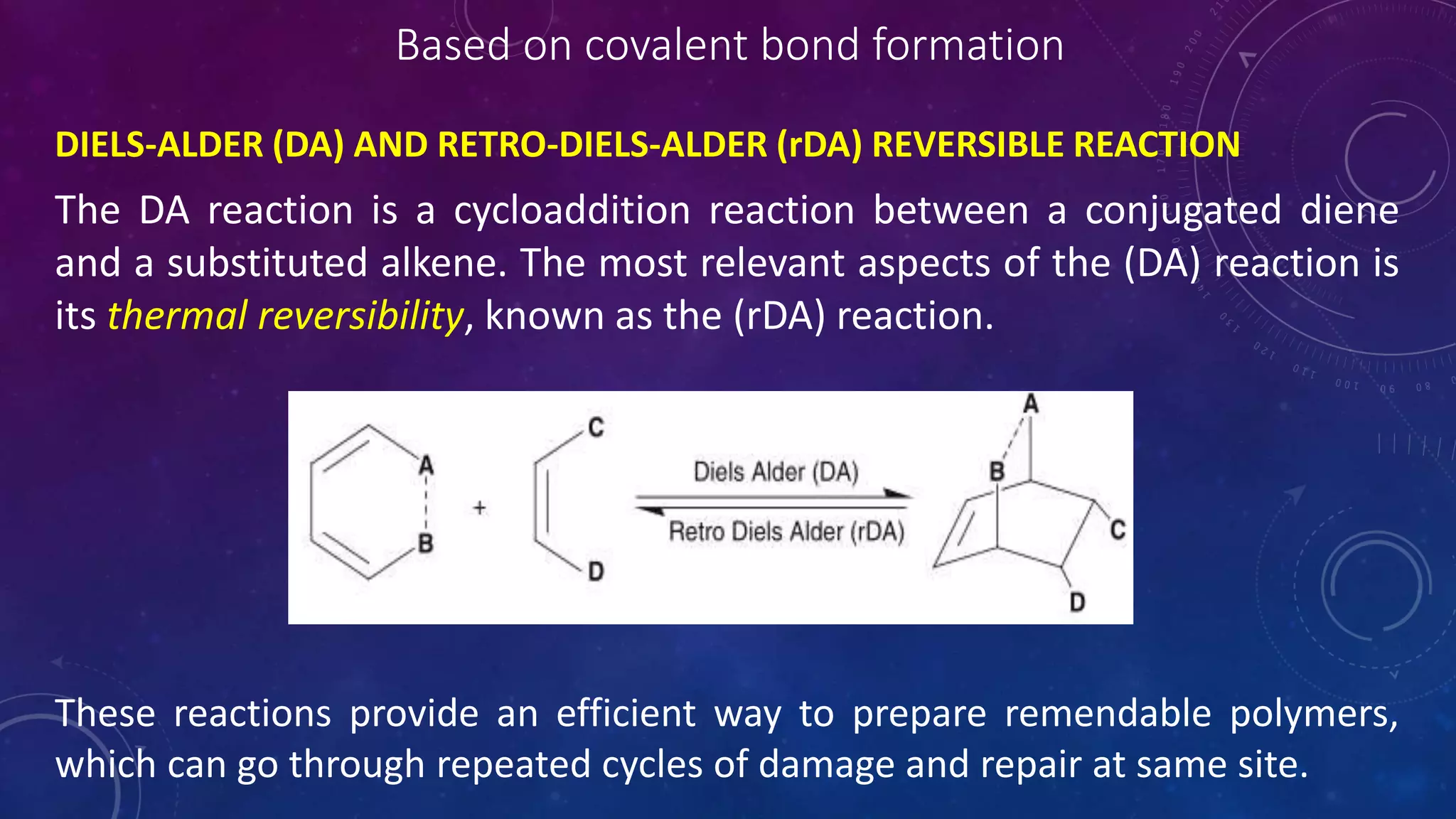
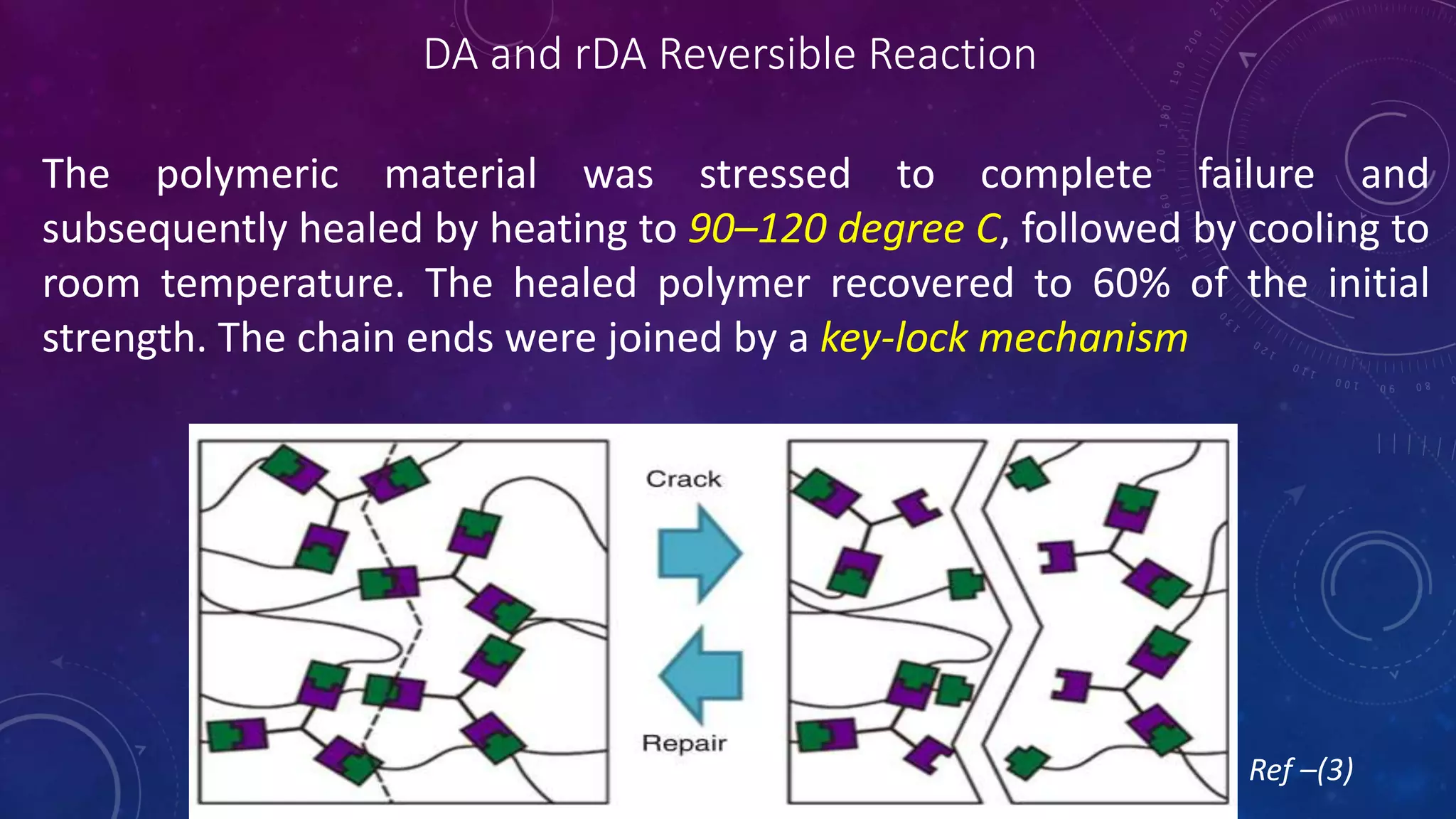
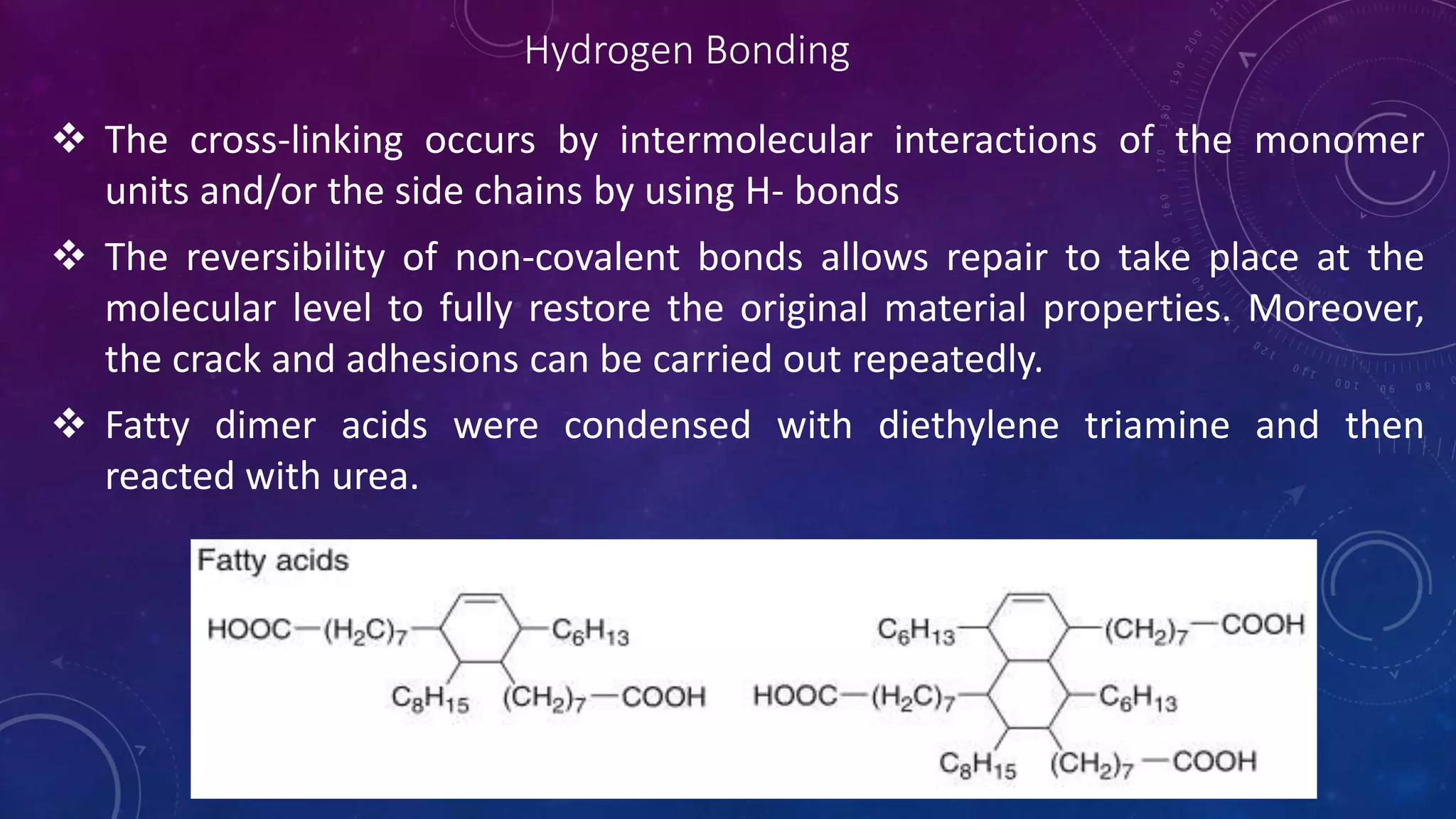
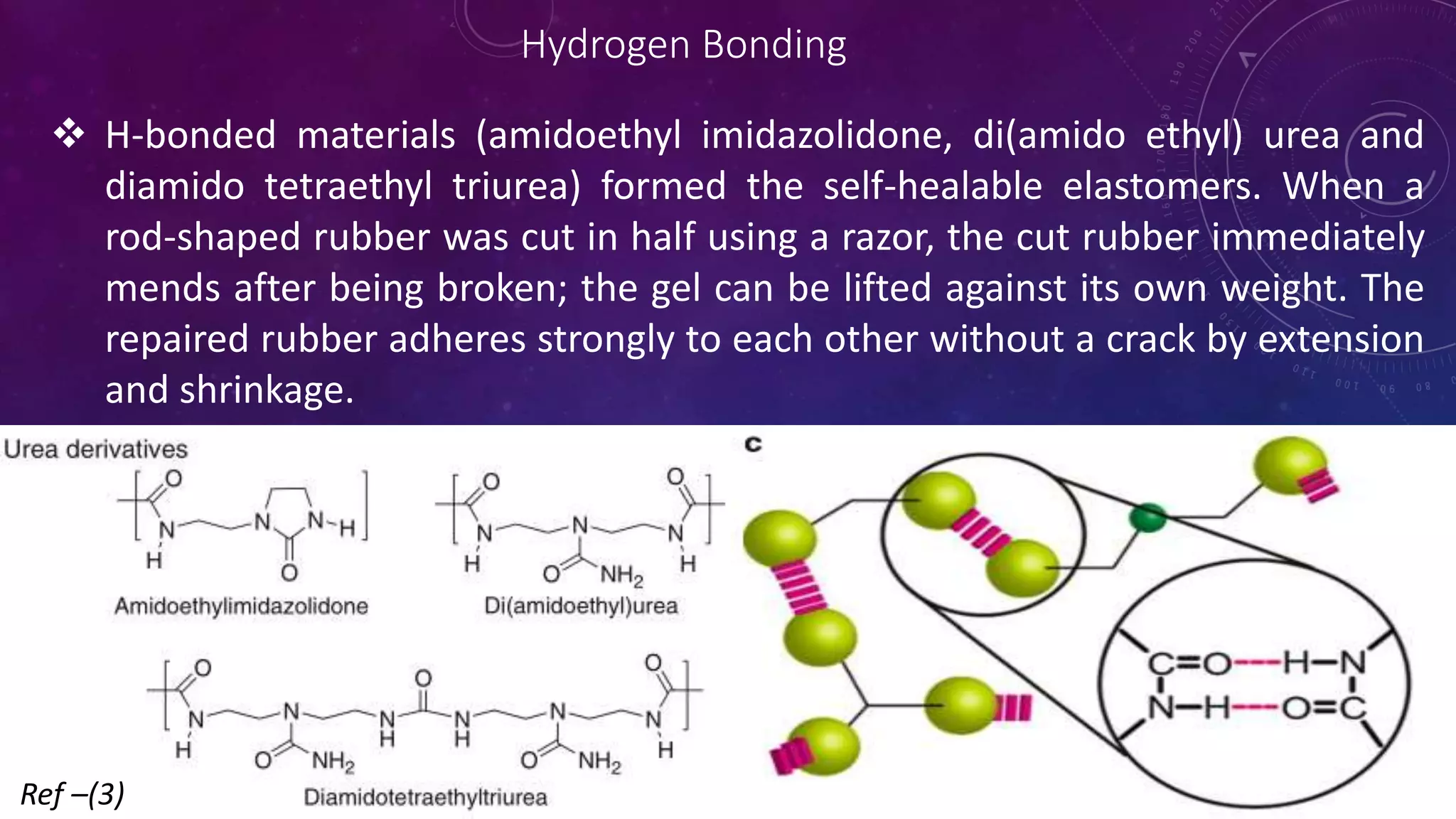
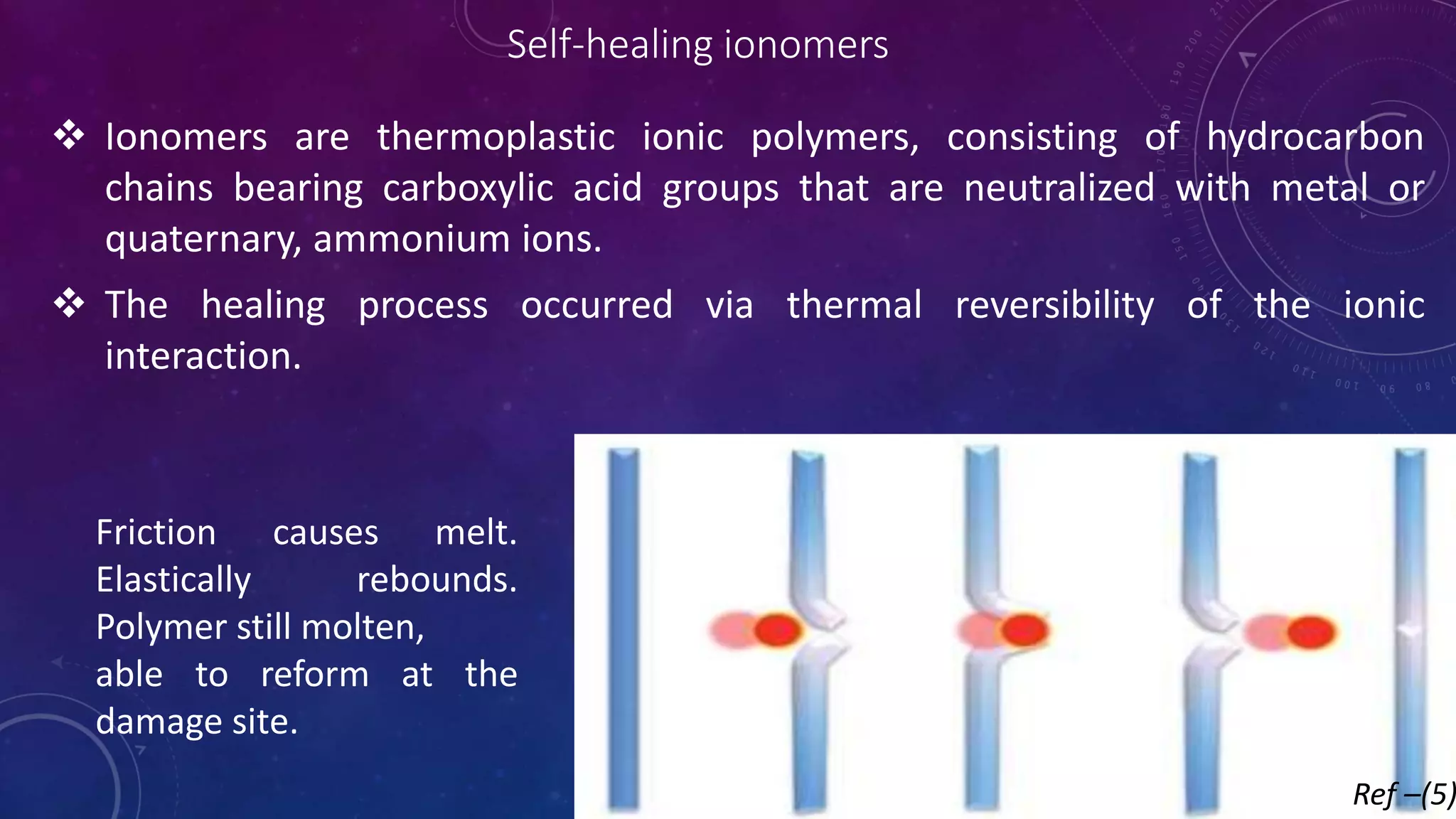
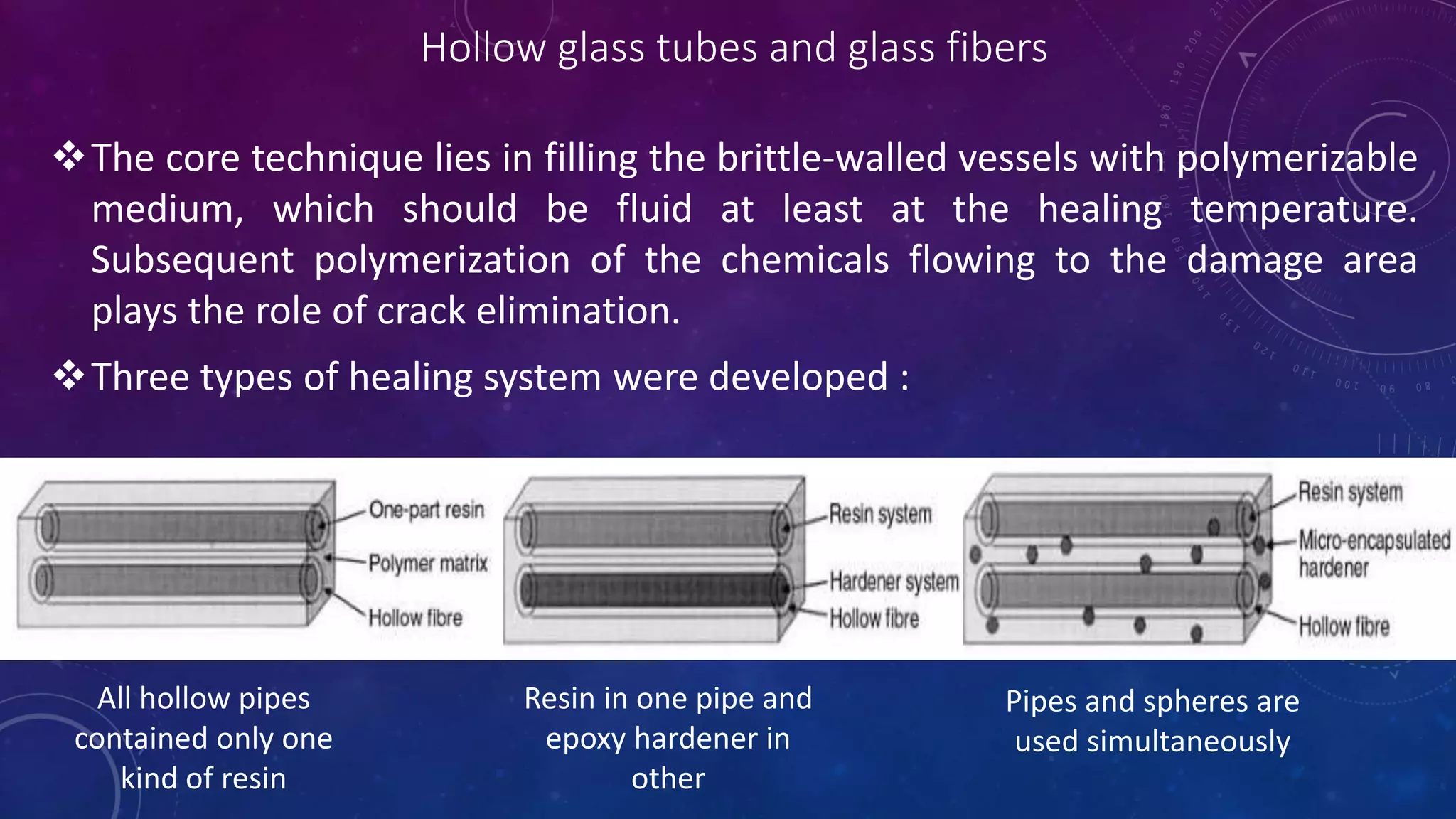
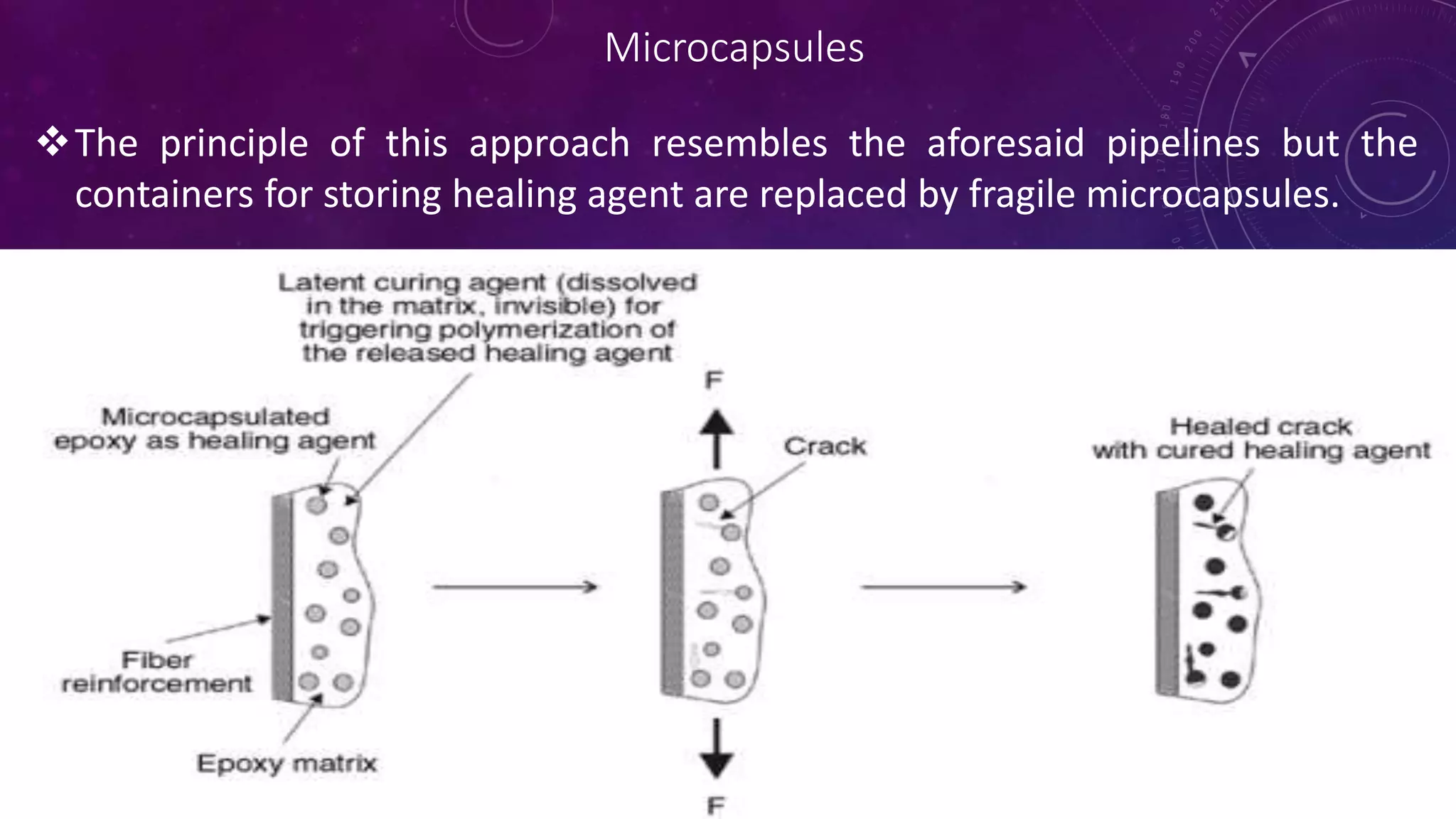
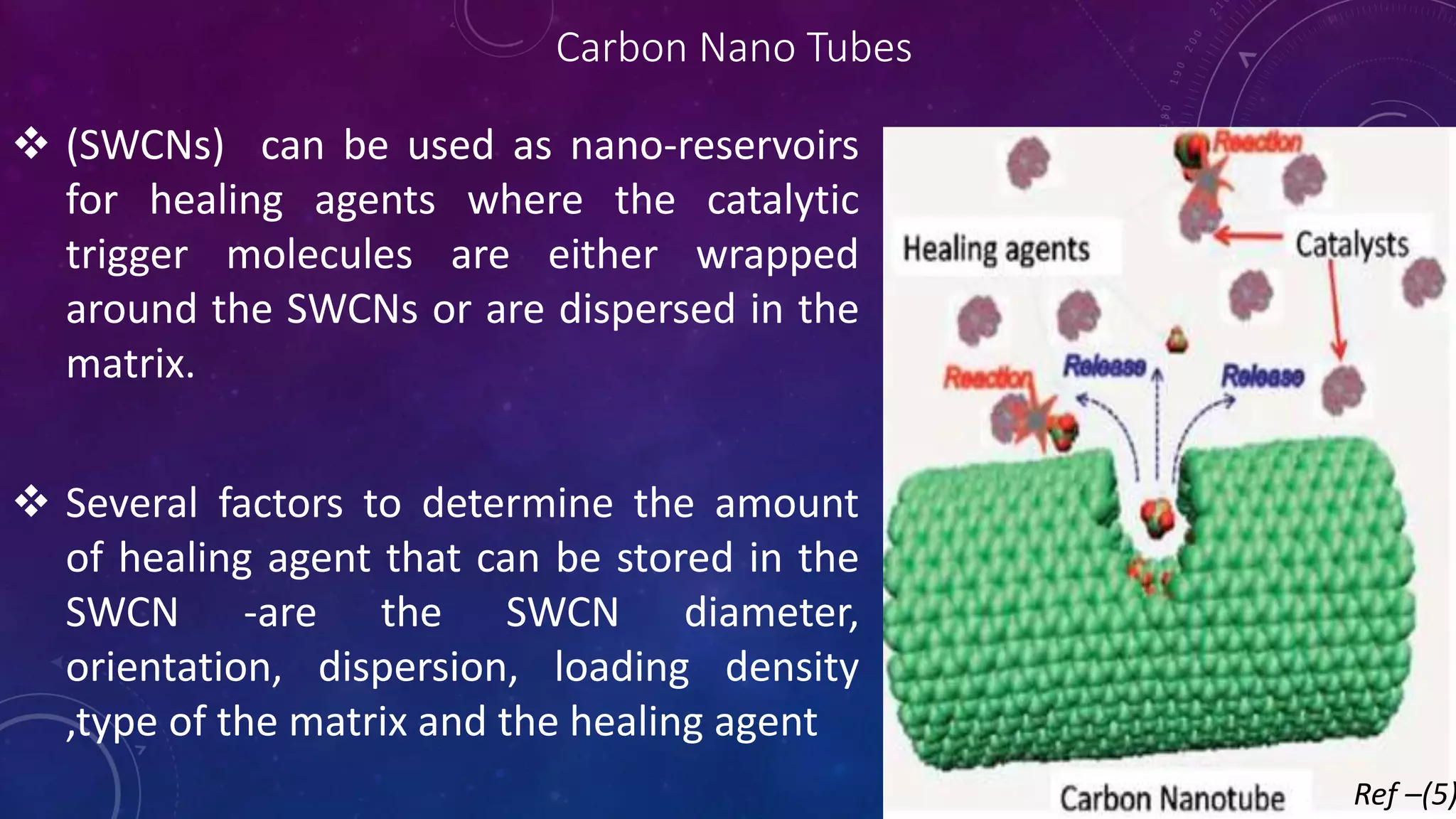
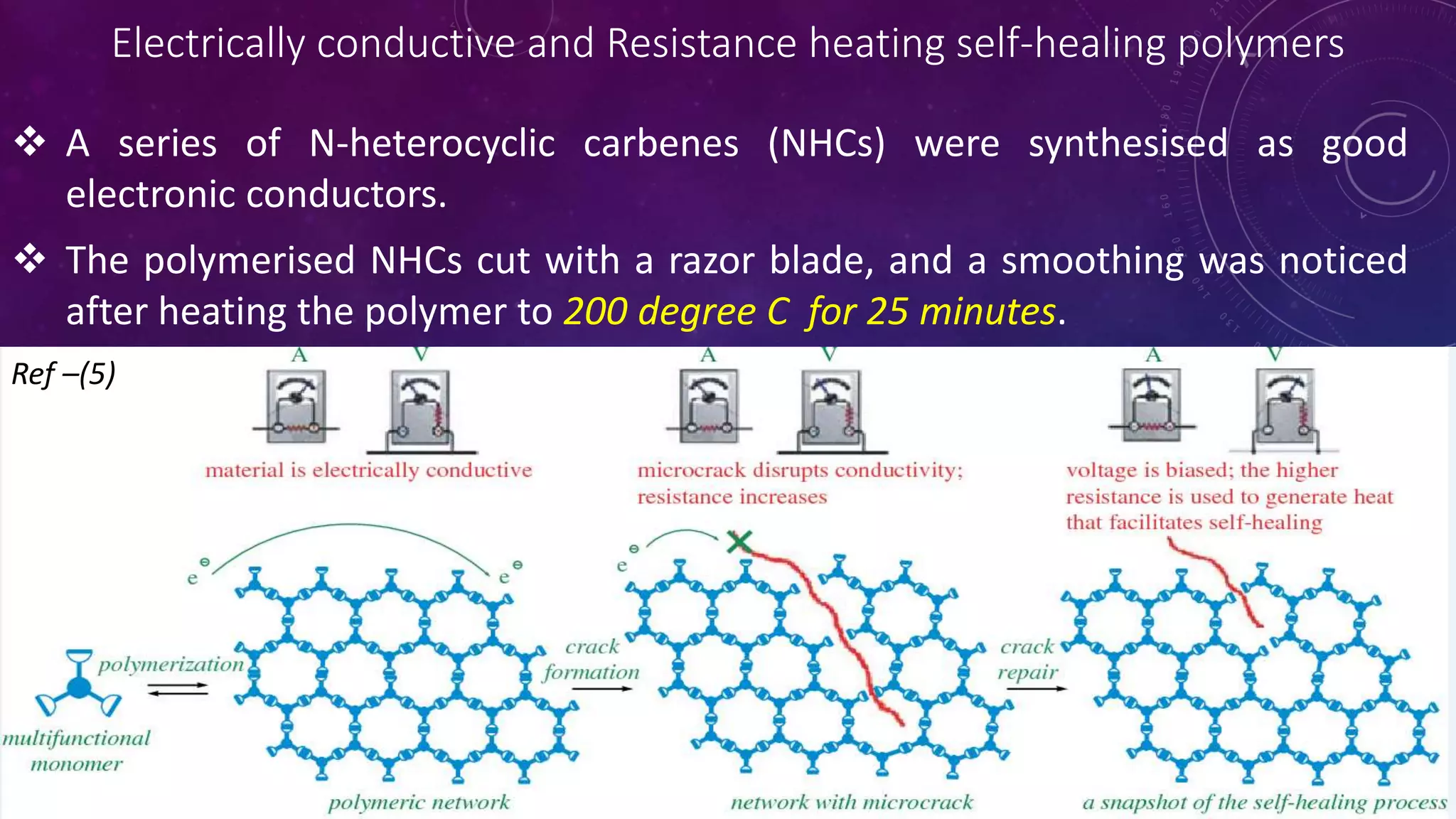
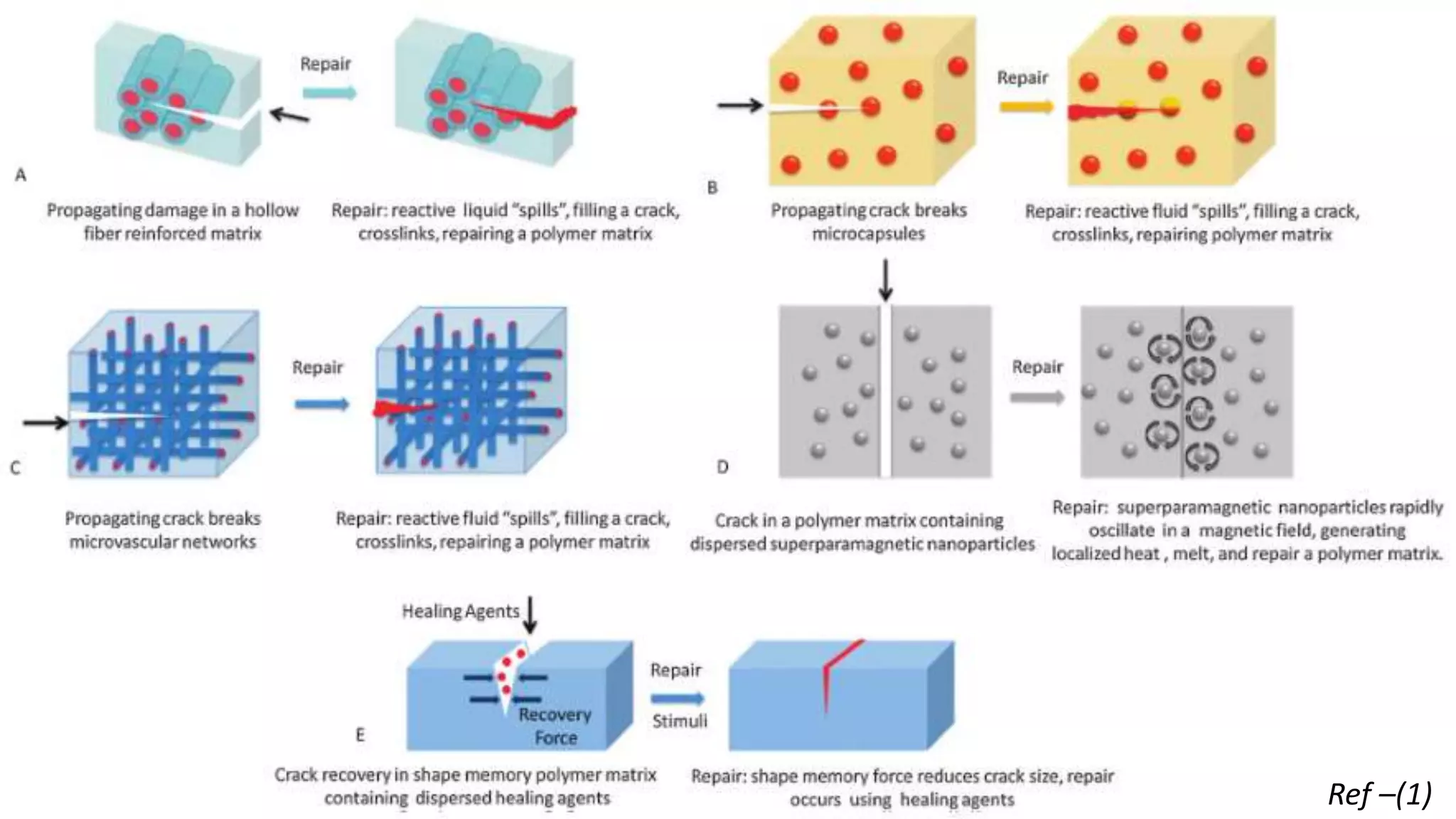
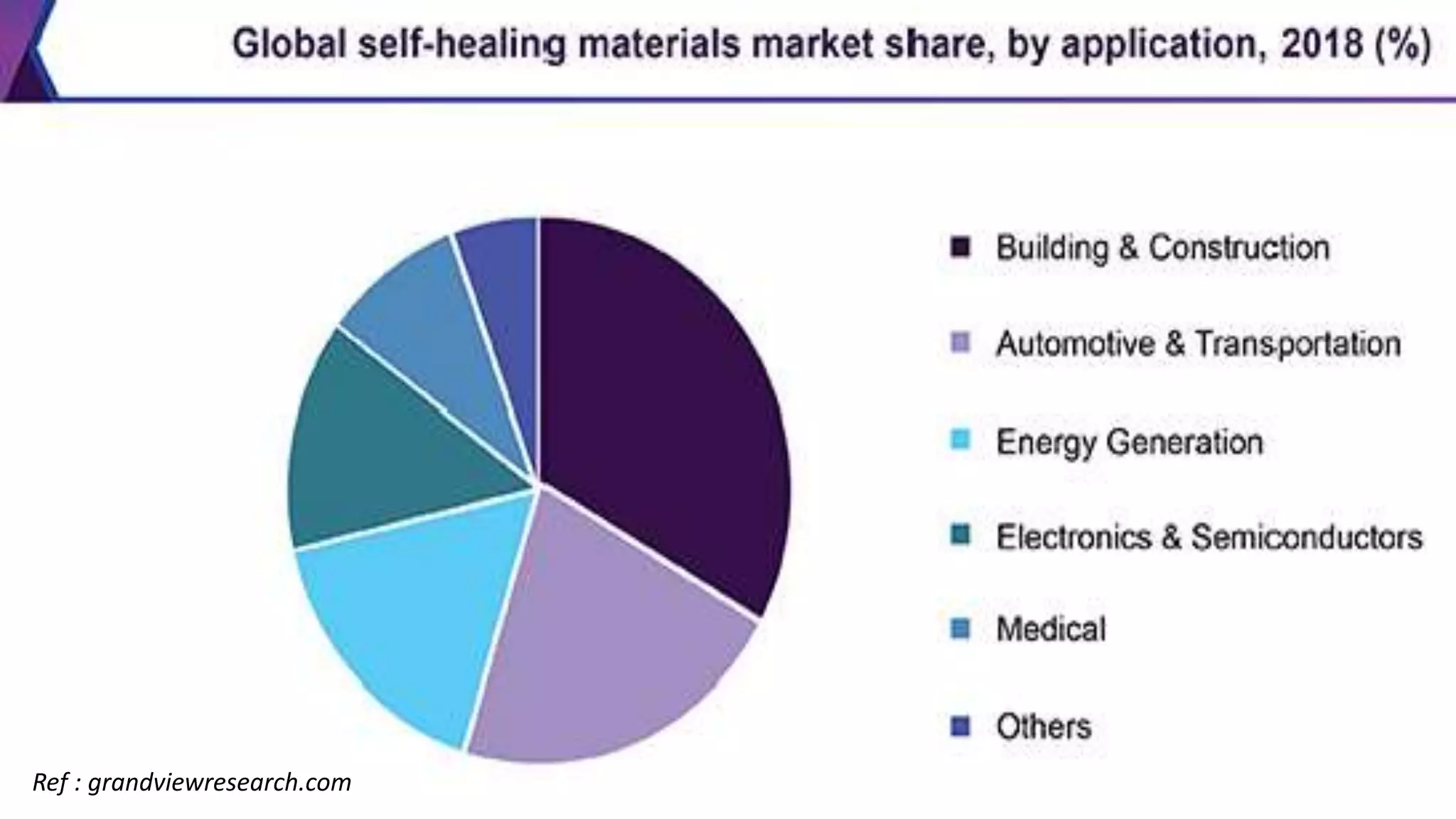
The document provides an overview of self-healing polymers, highlighting their ability to autonomously repair damage inspired by biological systems. It discusses intrinsic and extrinsic healing mechanisms, thermodynamics, applications in various fields, and challenges faced in the development of these materials. The conclusion emphasizes the need for new polymer designs to enhance durability and commercialization efforts.




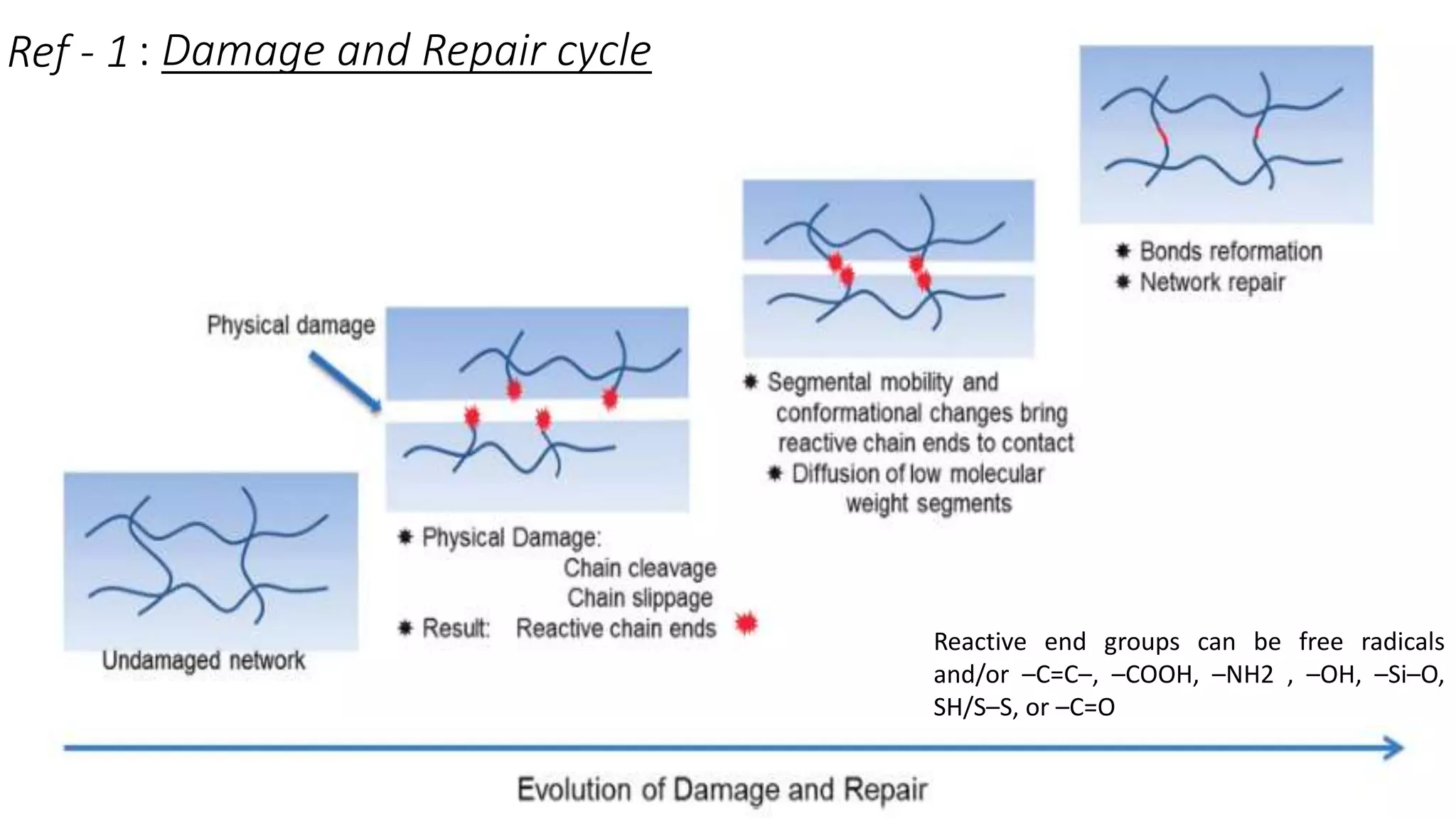
![Thermodynamics of self-healing
The self- repairing process can be viewed as series of chain conformational
changes and this approach provided the molecular weight (M) dependence of
repair time : (Tr) ∝ M3 implying that lower molecular weight polymers exhibit
favorable repairing conditions.
Fracture stress (s) is related to M and (Tr) via : (s) ∝ (Tr/M) 1/4
Healing efficiency R(s) can be defined by the extent of the recovery with
respect to its initial post-damage state as the ratio of fracture stress after and
before healing R(s) = [s Healed / s Initial ]](https://image.slidesharecdn.com/selfhealingpolymers-191026205633/75/An-Introduction-to-Self-healing-polymers-6-2048.jpg)